��Ŀ����
��7�֣���֪Ca(OH)2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ(�����ķ�Ӧ��Ϊ���ȷ�Ӧ)���������к���Cl ��ClO
��ClO ��
�� �����ֺ���Ԫ�ص����ӣ�����C1O
�����ֺ���Ԫ�ص����ӣ�����C1O ��
�� �������ӵ����ʵ���(n)�뷴Ӧʱ��(t)����������ͼ��ʾ��
�������ӵ����ʵ���(n)�뷴Ӧʱ��(t)����������ͼ��ʾ��

��1��t1ǰ������������ (�ѧʽ)��
(2)t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽΪ��
(3)��ʯ�����к���Ca(OH)2�����ʵ����� mol
(4)NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ�������� (����ĸ)��
(5)��ƽ�������ӷ���ʽ�� Fe(OH)3+ ClO + OH
+ OH ��������
��������



 ��ClO
��ClO ��
�� �����ֺ���Ԫ�ص����ӣ�����C1O
�����ֺ���Ԫ�ص����ӣ�����C1O ��
�� �������ӵ����ʵ���(n)�뷴Ӧʱ��(t)����������ͼ��ʾ��
�������ӵ����ʵ���(n)�뷴Ӧʱ��(t)����������ͼ��ʾ��
��1��t1ǰ������������ (�ѧʽ)��
(2)t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽΪ��
(3)��ʯ�����к���Ca(OH)2�����ʵ����� mol
(4)NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ�������� (����ĸ)��
| A��NaCl��Cl2 | B��NaCl��NaClO |
| C��NaClO3��NaClO4 | D��NaCl��NaClO3 |
 + OH
+ OH ��������
�������� 


��1��Ca(ClO)2 (1��)
(2) (2��)
(2��)
(3)5 (1��) (4)D (1��) (5)2, (5-n), 2n, (5-n) (n+3) (2��)
(2)
 (2��)
(2��)(3)5 (1��) (4)D (1��) (5)2, (5-n), 2n, (5-n) (n+3) (2��)
�����������1��Ca(ClO)2��t1ʱ�䣬Cl2ֻ��ʧȥ����������ClO-
(2)
 ���ݵ�ʧ�����غ������ƽ�����ClO-��ClO3-�ı�������2��1�����������Ԫ���غ��жϣ������ļ�̬�������ߵ�+1�ۺ�+3�ۣ����н��͵�-1�ۣ�����������Cl-��
���ݵ�ʧ�����غ������ƽ�����ClO-��ClO3-�ı�������2��1�����������Ԫ���غ��жϣ������ļ�̬�������ߵ�+1�ۺ�+3�ۣ����н��͵�-1�ۣ�����������Cl-��(3)���ݷ���ʽ���㣬��ͼ���֪ClO-Ϊ2mol �������������������Ca(OH)2�����ʵ�����5mol��
��4��NaClO2���ȵļ�̬��+3�ۣ������绯��Ӧ��������̬���� NaClO3�ֽ��ͼ�̬����NaCl��
(5)���ݵ�ʧ�����غ�����ƽ�����ȱ�������� FeO4n-��Fe�ļ�̬Ϊ8-n,����Fe�仯�ļ�̬Ϊ8-n-3����5-n����Ӧ�����ȵļ�̬Ϊ+1�ۣ����ɵ���Ϊ-1�ۣ����Ա仯�ļ�̬Ϊ2��ȡ��С������2��5-n��,���Է�Ӧ������������ǰ����2������ǰ���䣨5-n��,ClO-ǰ���䣨5-n����������OԪ���غ�,HԪ���غ㣬OH
 ǰ����2n��
ǰ����2n��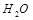 ǰ����n+3��
ǰ����n+3�����������⿼��Ƚ��ۺϣ��ѵ��������ӷ�Ӧ����ʽ����д���Լ�����������ԭ��Ӧ�е�ʧ�����غ��ԭ����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 4NH3����3SO2����N2����6H2O����Ӧ�����ɵ���������ͻ�ԭ��������ʵ���֮����
4NH3����3SO2����N2����6H2O����Ӧ�����ɵ���������ͻ�ԭ��������ʵ���֮����