��Ŀ����
�����£�������Һ�е���Ũ�ȹ�ϵ��ȷ����(����)
2c(H��)��2c(OH��)��c(CH3COO��)��c(CH3COOH)[
| A��������ˮ�м������NaOH��c(Na��)��c(Cl��)��c(ClO��)��c(OH��) |
| B��pH��8.3��NaHCO3��Һ��c(Na��)��c(HCO3-)��c(CO32-)��c(H2CO3) |
| C��pH��11�İ�ˮ��pH��3������������ϣ�c(Cl��)��c(NH4+)��c(OH��)��c(H��) |
| D��0.2 mol��L��1 CH3COOH��Һ��0.1 mol��L��1 NaOH��Һ�������ϣ� |
D
���������A�����ݵ���غ��֪��c(H+) +c(Na��)��c(Cl��)��c(ClO��)��c(OH��).����B��pH��8.3��NaHCO3��Һ��HCO3-��ˮ�����ô��ڵ������á�����c(Na��)��c(HCO3-)��c(H2CO3) ��c(CO32-)������C��pH��11�İ�ˮ��pH��3������C(H��)=C(OH��)=10-3mol/L,����һˮ�ϰ������������ǿ�ᣬ����c(��ˮ)�� c(HCl) =10-3mol/L.�������Ϻ����ڰ�ˮ����������c(NH4+)��c(Cl��) ��c(OH��) ��c(H��)������D��0.2 mol��L��1 CH3COOH��Һ��0.1 mol��L��1 NaOH��Һ�������ϣ�������ӦCH3COOH+NaOH= CH3COONa + H2O�����������غ�ɵâ�c(CH3COO��)+c(CH3COOH) ��2c(Na+).���ݵ���غ�ɵã�c(CH3COO��)+ c(OH��)��c(H��)+c(Na+)���� c(Na+)= c(CH3COO��)+=c(OH��)- c(H��)����=�ڴ����ʽ�ã�2c(H��)��2c(OH��)��c(CH3COO��)��c(CH3COOH) ����ȷ��

��ϰ��ϵ�д�
�����Ŀ
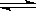 H3O++CO32��
H3O++CO32�� HCO3-��OH����ͨ��CO2��ƽ�⳯����Ӧ�����ƶ�
HCO3-��OH����ͨ��CO2��ƽ�⳯����Ӧ�����ƶ�