��Ŀ����
������Һ�и�����Ũ�ȹ�ϵ��ȷ����
| A��0.1 mol��L��1 NaHSO4��Һ�У�c(Na��)��c(SO42-)��c(H��) ��c(OH��) |
| B��0.1 mol��L��1Na2S��Һ�У�2 c(Na��)��c(S2��)��c(HS��)��c(H2S) |
| C��0.1 mol��L��1 NaHCO3��Һ�У�c(Na��)��c(H��)��c(HCO3-)��2c(CO32-)��c(OH��) |
| D��������������ʵ���Ũ�ȵ�������Һ������������Һ��Ϻ�C(Na��)��c(CH3COO��)��c(H��)��c(OH��) |
C
���������A��0.1 mol��L��1 NaHSO4��Һ�У�c(Na��)��c(SO42-)��c(H��) ��c(OH��)������B��0.1 mol��L��1Na2S��Һ�У�
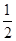 c(Na��)��c(S2��)��c(HS��)��c(H2S)������C������غ㣬��ȷ��D��������������ʵ���Ũ�ȵ�������Һ������������Һ��Ϻ����ɴ�������Һ����������ӻ�ˮ�⣬�������¹�ϵ��C(Na��)��c(CH3COO��)��c(OH��) ��c(H��)������
c(Na��)��c(S2��)��c(HS��)��c(H2S)������C������غ㣬��ȷ��D��������������ʵ���Ũ�ȵ�������Һ������������Һ��Ϻ����ɴ�������Һ����������ӻ�ˮ�⣬�������¹�ϵ��C(Na��)��c(CH3COO��)��c(OH��) ��c(H��)������
��ϰ��ϵ�д�
�����Ŀ
 H++B2��
H++B2�� S2-��H3O+
S2-��H3O+ 2H+ +Y2-
2H+ +Y2-