��Ŀ����
ij���������ð�������Ĵ����к��������Ȼ������ʣ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�����������ʵ�飺ȡ16.5g������Ʒ�����ձ��У����ձ����ڵ�����ƽ�ϣ��ٰ�150.0gϡ���ᣨ������������Ʒ�У��۲�����仯���±���ʾ��| ʱ��/s | 5 | 10 | 15 | |
| ����/g | 215.2 | 211.4 | 208.6 | 208.6 |
��1��ʵ���в�����CO2��������Ϊ______��
��2���ò�Ʒ��̼���Ƶ��������� �������ȷ��С�����һλ��______��
��2������Na2CO3��CO2�����ݶ�����̼����������̼���Ƶ��������ٸ�����������������㣮
����⣺��1���ɱ������ݿ�֪��10sʱ����Ӧ��ȫ����������Ϊ���ɶ�����̼�������������ɶ�����̼������Ϊ215.2g-208.6g=6.6g��
�ʴ�Ϊ��6.6g��
��2������6.6g������̼����
Na2CO3��������CO2
106 44
m��Na2CO3�� 6.6g
��m��Na2CO3��=6.6g×
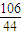 =15.9g
=15.9g����Ʒ��̼���Ƶ���������
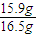 ×100%=96.4%��
×100%=96.4%���ʴ�Ϊ��96.4%��
���������⿼���йػ�ѧ���㣬�ѶȲ���ȷ��������̼�������ǹؼ���ע����������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�(5��)ij���������ð�������Ĵ����к��������Ȼ������ʡ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�����������ʵ�飺ȡ16��5 g������Ʒ�����ձ��У����ձ����ڵ��ӳ��ϣ��ٰ�150��0 gϡ����(����)������Ʒ�С��۲�����仯���±���ʾ��
| ʱ�䣯s | 0 | 5 | 10 | 15 |
| ������g | 215��2 | 211��4 | 208��6 | 208��6 |
����ݴ˷������㣺
(1)�ò�Ʒ��̼���Ƶ���������?(�����ȷ��0��l��)
(2)����Ӧ����ҺΪ150��0 mL��������Һ��NaCI���ʵ���Ũ�ȡ�
ij���������ð�������Ĵ����к��������Ȼ������ʡ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�����������ʵ�飺ȡ16.5 g������Ʒ�����ձ��У����ձ����ڵ�����ƽ�ϣ��ٰ�150.0 gϡ����(����)������Ʒ�С��۲�����仯���±���ʾ��
| ʱ�䣯s | 0 | 5 | 10 | 15 |
| ������g | 215.2 | 211.4 | 208.6 | 208.6 |
��1��ʵ���в�����CO2��������Ϊ ��
��2���ò�Ʒ��̼���Ƶ��������� (�����ȷ��С�����һλ) ��
ij���������ð�������Ĵ����к��������Ȼ������ʡ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�����������ʵ�飺ȡ16.5 g������Ʒ�����ձ��У����ձ����ڵ�����ƽ�ϣ��ٰ�150.0 gϡ����(����)������Ʒ�С��۲�����仯���±���ʾ��
|
ʱ�䣯s |
0 |
5 |
10 |
15 |
|
������g |
215.2 |
211.4 |
208.6 |
208.6 |
����ݴ˷������㣺
��1��ʵ���в�����CO2��������Ϊ ��
��2���ò�Ʒ��̼���Ƶ��������� (�����ȷ��С�����һλ) ��
(5��)ij���������ð�������Ĵ����к��������Ȼ������ʡ�Ϊ�˲ⶨ�ò�Ʒ��̼���ƵĴ��ȣ�����������ʵ�飺ȡ16��5 g������Ʒ�����ձ��У����ձ����ڵ��ӳ��ϣ��ٰ�150��0 gϡ����(����)������Ʒ�С��۲�����仯���±���ʾ��
|
ʱ�䣯s |
0 |
5 |
10 |
15 |
|
������g |
215��2 |
211��4 |
208��6 |
208��6 |
����ݴ˷������㣺
(1)�ò�Ʒ��̼���Ƶ���������?(�����ȷ��0��l��)
 (2)����Ӧ����ҺΪ150��0 mL��������Һ��NaCI���ʵ���Ũ�ȡ�
(2)����Ӧ����ҺΪ150��0 mL��������Һ��NaCI���ʵ���Ũ�ȡ�