��Ŀ����
����Ŀ����ҵ�Ͽ������ﴦ����KCN�ķ�ˮ����һ������������������������£��� KCNת����KHCO3�� NH3( ��� pH:6.7��7.2)���ڶ����ǰѰ�ת��Ϊ���NH3+2O2![]() HNO3+H2O,���������գ�
HNO3+H2O,���������գ�
(1)д����һ����Ӧ�Ļ�ѧ����ʽ__________________________��
(2)����ڶ�����Ӧ�е���ת�Ƶķ������Ŀ��NH3+2O2![]() HNO3+H2O__________д������ˮ�ĵ���ʽ_________��
HNO3+H2O__________д������ˮ�ĵ���ʽ_________��
(3)����KHCO3���ڻ�����________��ѡ����ӡ����ۡ�), �������ڶ�������ԭ�Ӱ뾶����Ԫ����___________����Ԫ�ط��ţ���KHCO3 ��ˮ��ҺpH_____7 ��ѡ���������=������)��
(4)д��һ���ܱȽ� KCN��̼Ԫ�غ͵�Ԫ�طǽ�����ǿ����ʵ����ʵ��__________��
(5)��ҵ�ϻ�������������������KCN �ķ�ˮ��
KCN+2KOH+Cl2=KOCN+2KCl+ H2O
2KOCN+4KOH+3Cl2=N2 +6KCl+2CO2+ 2H2O
�Ƚ����ﴦ������������������ȱ�㣨��дһ�㣩��_______��
���𰸡�2KCN +O2+ 4H2O 2KHCO3 +2NH3
2KHCO3 +2NH3 ![]()
 HNO3 +H2O
HNO3 +H2O ![]() ���� C �� ̼��������ϡ���ᷴӦ����������̼���� �ŵ㣺����������й©�ķ��գ�ȱ�㣺������Ӧ�Բ��Ϊ������ʧ��Ի���Ҫ���
���� C �� ̼��������ϡ���ᷴӦ����������̼���� �ŵ㣺����������й©�ķ��գ�ȱ�㣺������Ӧ�Բ��Ϊ������ʧ��Ի���Ҫ���
��������
��1������������ã�KCN��O2��H2O��Ӧ����KHCO3��NH3���䷴Ӧ����ʽΪ2KCN +O2+ 4H2O 2KHCO3 +2NH3���ʴ�Ϊ�� 2KCN +O2+ 4H2O
2KHCO3 +2NH3���ʴ�Ϊ�� 2KCN +O2+ 4H2O 2KHCO3 +2NH3��
2KHCO3 +2NH3��
��2���ڶ��������ķ�Ӧ����ʽΪ��NH3+2O2 HNO3+H2O���÷�Ӧ�У������е�Ԫ�ػ��ϼ۴�-3������Ϊ+5�ۣ�ʧȥ8�����ӣ���������Ԫ�صĻ��ϼ۴�0�۽���Ϊ-2�ۣ��ܹ��õ�8�����ӣ������Ӧ�е���ת�Ƶķ������Ŀ��
HNO3+H2O���÷�Ӧ�У������е�Ԫ�ػ��ϼ۴�-3������Ϊ+5�ۣ�ʧȥ8�����ӣ���������Ԫ�صĻ��ϼ۴�0�۽���Ϊ-2�ۣ��ܹ��õ�8�����ӣ������Ӧ�е���ת�Ƶķ������Ŀ��![]()
 HNO3 +H2O������ˮ�ĵ���ʽΪ��
HNO3 +H2O������ˮ�ĵ���ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]()
 HNO3 +H2O��
HNO3 +H2O��![]() ��
��
��3������KHCO3�������ӻ������ǿ�������Σ���ˮ��Һ�Լ��ԣ�pH��7�� KHCO3�к���K��H��C��O����Ԫ�أ����ڶ����ڵ���H��C��O����Ԫ�أ�ͬ���ڣ�������ԭ�Ӱ뾶��С������ԭ�Ӱ뾶����Ԫ����C���ʴ�Ϊ�����ӣ�C������
��4��ϡ������̼�����Ʒ�Ӧ���������ơ�������̼��ˮ���仯ѧ��Ӧ����ʽΪ��HNO3+NaHCO3=NaNO3+H2O+CO2����˵����Ԫ�صķǽ�����ǿ��̼Ԫ�صķǽ����ԣ��ʴ�Ϊ��̼��������ϡ���ᷴӦ����������̼���塣
��5��������ã����ﴦ�������ŵ㣺����������й©�ķ��յȣ�ȱ�㣺������Ӧ�Բ��Ϊ������ʧ��Ի���Ҫ��ߵȣ��ʴ�Ϊ������������й©�ķ��գ�������Ӧ�Բ��Ϊ������ʧ��Ի���Ҫ��ߵȡ�

����Ŀ��������������![]() ���ı���Ϊ��Ϣ��������������һ����ɫ��Һ�壬������ˮ���з�����ζ������������ˮ���㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��塢�ܼ��ȡ����Ʊ�����Ϊ��
���ı���Ϊ��Ϣ��������������һ����ɫ��Һ�壬������ˮ���з�����ζ������������ˮ���㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��塢�ܼ��ȡ����Ʊ�����Ϊ��
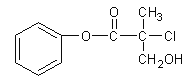
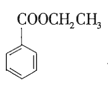
 +CH3CH2OH
+CH3CH2OH![]()
 +H2O
+H2O
��֪����������100���Ѹ������������л�������������ʾ��
���� | ��Է������� | ��ɫ��״̬ | �е�/�� | �ܶ�/�� |
������ | 122 | ��ɫ��Ƭ״����״���� | 249 | 1.2659 |
���������� | 150 | ��ɫ����Һ�� | 212.6 | 1.05 |
�Ҵ� | 46 | ��ɫ����Һ�� | 78.3 | 0.7893 |
������ | 84 | ��ɫ����Һ�� | 80.7 | 0.78 |
ʵ�鲽�����£�
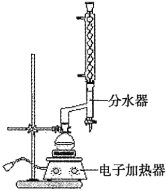
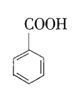
����Բ����ƿ�м���![]() �����ᣬ
�����ᣬ![]() �Ҵ�����������20mL�������Լ�4mLŨ���ᣬ��Ͼ��Ȳ������ʯ������ͼ��ʾװ��װ�������������¶���65~70����Ȼ���2h�����÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
�Ҵ�����������20mL�������Լ�4mLŨ���ᣬ��Ͼ��Ȳ������ʯ������ͼ��ʾװ��װ�������������¶���65~70����Ȼ���2h�����÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
�ڷ�Ӧ�������������ų���ˮ���е�Һ��ر������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȡ�
�۽���ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У���������![]() ����Һ�����ԡ��÷�Һ©���ֳ��л��㣬ˮ����25mL������ȡ��Һ��Ȼ��ϲ����л��㣬�����Ȼ��ƣ����ã����ˣ�����Һ�����������������Ѻͻ�����������£�����210~213�����֡�
����Һ�����ԡ��÷�Һ©���ֳ��л��㣬ˮ����25mL������ȡ��Һ��Ȼ��ϲ����л��㣬�����Ȼ��ƣ����ã����ˣ�����Һ�����������������Ѻͻ�����������£�����210~213�����֡�
�ܼ���ϸ�ò�Ʒ���Ϊ![]() ��
��
�ش��������⣺
��1���ڸ�ʵ���У�Բ����ƿ���ݻ����ʺϵ���________������ţ���
A.25mL B.50mL C.100mL D.250mL
��2���������ʹ�÷�ˮ�����Ϸ����ȥˮ��Ŀ����______________��
��3���������Ӧ���Ƽ���������¶�Ϊ________������ţ���
A.65~70�� B.78~80�� C.85~90�� D.215~220��
��4������ۼ���![]() ��������________________________________����
��������________________________________����![]() �ļ��������㣬��֮������ʱ��������ƿ�пɼ����������ɣ������������ԭ����__________________��
�ļ��������㣬��֮������ʱ��������ƿ�пɼ����������ɣ������������ԭ����__________________��
��5�����ڲ�����е���ȡ��Һ������������ȷ��________������ţ���
A.ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ���������Һ©����ת������������ҡ
B.��ҡ���κ����Һ©���ϿڵIJ���������
C.��������ҡ���������ֳַ�Һ©�����ô�Һ��ֲ�
D.�ų�Һ��ʱ��Ӧ���Ͽڲ������������ϵİ��۶�©�����ϵ�С��
��6������ɵñ�ʵ��IJ���Ϊ________��