��Ŀ����
����ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
��1�����ڸ÷�Ӧ������˵���У���ȷ����______��
A����H��0����S��0��B����H��0����S��0��
C����H��0����S��0��D����H��0����S��0��
��2���÷�Ӧƽ�ⳣ��K�ı���ʽΪ______��
��3���¶Ƚ��ͣ�ƽ�ⳣ��K______������������䡱��С������
��4��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1L���ܱ������У�����1molCO2��3molH2�����CO2��CH3OH��g����Ũ����ʱ��仯����ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v��H2��______mol?L-1?min-1��

��5�����д�ʩ����ʹ
�������______��
A�������¶ȣ�B�����������
C����H2O��g������ϵ�з��룻D������He��g����ʹ��ϵ��ѹǿ����

��1�����ڸ÷�Ӧ������˵���У���ȷ����______��
A����H��0����S��0��B����H��0����S��0��
C����H��0����S��0��D����H��0����S��0��
��2���÷�Ӧƽ�ⳣ��K�ı���ʽΪ______��
��3���¶Ƚ��ͣ�ƽ�ⳣ��K______������������䡱��С������
��4��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1L���ܱ������У�����1molCO2��3molH2�����CO2��CH3OH��g����Ũ����ʱ��仯����ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v��H2��______mol?L-1?min-1��

��5�����д�ʩ����ʹ
| n(CH3OH) |
| n(CO2) |
A�������¶ȣ�B�����������
C����H2O��g������ϵ�з��룻D������He��g����ʹ��ϵ��ѹǿ����

��1����ͼ���֪����Ӧ��������ߣ�������������ͣ��÷���Ϊ���ȷ�Ӧ���ʡ�H��0����Ӧ�����������С����S��0���ʴ�Ϊ��C��
��2���÷�Ӧƽ�ⳣ��K�ı���ʽΪ
���ʴ�Ϊ��
��
��3���¶Ƚ��ͣ�ƽ������ȵķ����ƶ�������Ӧ��������ƽ�ⳣ�����ʴ�Ϊ������
��4����ͼ���֪v��CO2��=
=0.075mol/��L?min�������ݷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȿ�֪v��H2��=3v��CO2��=3��0.075mol/��L?min��=0.225 mol/��L?min�����ʴ�Ϊ��0.225 mol/��L?min����
��5��A�������¶ȣ�ƽ�����淴Ӧ�����ƶ����״��������̼�����ʵ�����ֵ��С����A����
B�����������ƽ�ⲻ�ƶ������߱�ֵ���䣬��B����
C����H2O��g������ϵ�з��룬�������Ũ�ȼ�С��ƽ��������Ӧ�����ƶ������߱�ֵ���C��ȷ��
D������He��g����ʹ��ϵ��ѹǿ����ƽ�ⲻ�ƶ�����D����
�ʴ�Ϊ��C��
��2���÷�Ӧƽ�ⳣ��K�ı���ʽΪ
| c(CH3OH)?c(H2O) |
| c(CO2)?c2(H2)2 |
| c(CH3OH)?c(H2O) |
| c(CO2)?c2(H2) |
��3���¶Ƚ��ͣ�ƽ������ȵķ����ƶ�������Ӧ��������ƽ�ⳣ�����ʴ�Ϊ������
��4����ͼ���֪v��CO2��=
| 0.75mol/L |
| 10min |
��5��A�������¶ȣ�ƽ�����淴Ӧ�����ƶ����״��������̼�����ʵ�����ֵ��С����A����
B�����������ƽ�ⲻ�ƶ������߱�ֵ���䣬��B����
C����H2O��g������ϵ�з��룬�������Ũ�ȼ�С��ƽ��������Ӧ�����ƶ������߱�ֵ���C��ȷ��
D������He��g����ʹ��ϵ��ѹǿ����ƽ�ⲻ�ƶ�����D����
�ʴ�Ϊ��C��

��ϰ��ϵ�д�
�����Ŀ
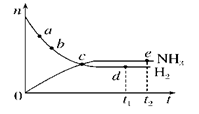
 2NH3(g)(����ӦΪ���ȷ�Ӧ)��673 K��30 MPa�£�n(NH3)��n(H2)��ʱ��t�仯�Ĺ�ϵʾ��ͼ��ͼ��ʾ��������������ȷ����(����)
2NH3(g)(����ӦΪ���ȷ�Ӧ)��673 K��30 MPa�£�n(NH3)��n(H2)��ʱ��t�仯�Ĺ�ϵʾ��ͼ��ͼ��ʾ��������������ȷ����(����)