��Ŀ����
����Ŀ������TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�á��Ʊ�����TiO2�ķ���֮һ��TiCl4ˮ������TiO2xH2O�������ˡ�ˮϴ��ȥ���е�Cl-���ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2����������ԭ�ζ����ⶨTiO2������������ȡ17.2gTiO2��Ʒ��һ���������ܽⲢ��ԭΪTi3+������Һ��ˮϡ�����250mL��Һ��ȡ��25.00mL����Һ����ƿ�У��μ�KSCN��Һ��ָʾ������0.5mol/L��NH4Fe(SO4)2����Һ�ζ�Ti3+��ȫ������Ti4+����ش��������⣺
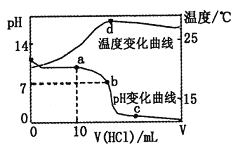
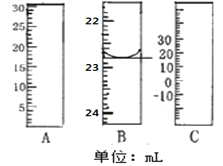
��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣�����A����������Ϊ________B����ȷ����Ϊ________��
��2��TiCl4ˮ������TiO2xH2O�Ļ�ѧ����ʽΪ________��
��3���жϵζ��յ��������_____________��
��4���ζ������յ�ʱ������ȥ0.5mol/L��NH4Fe(SO4)2����Һ40.00mL,��ԭ��Ʒ��TiO2��������______________��
��5���ж����в�����TiO2���������ⶨ�����Ӱ�죨����ƫ��������ƫ����������Ӱ��������
���������Ʊ���Һ�����У��ձ��е�NH4Fe(SO4)2��Һ������������ʹ�ⶨ���________��
�����ڵζ��յ��ȡ�ζ��ܶ���ʱ�����ӱ�ҺҺ�棬ʹ�ⶨ���__________��
���𰸡���Ͳ 22.80mL TiCl4��(2��x)H2O= TiO2��xH2O����4HCl �������һ��NH4Fe(SO4)2��Һ����Һ��ɺ�ɫ���Ұ���Ӳ��ָ�ԭ����ɫ 93% ƫ�� ƫ��
��������
��1�������ͼʾ��֪��A�����Ŀ̶��ϴ���С��Ϊ��Ͳ������B�Ŀ̶���С�´�Ϊ�ζ��ܣ�����C��0�̶ȣ�Ϊ�¶ȼƣ�
��2���������Ϣ��֪TiCl4����ˮ�ⷴӦ����TiO2xH2O���������
��3����NH4Fe(SO4)2����Һ�ζ�ʱ��Fe3+��Ti3+����������ԭ��Ӧ��Ti3+��������������Ti4+��Fe3+����ԭΪFe2+�����ﵽ�ζ��յ�ʱ���ټ���NH4Fe��SO4��2��Һ����Һ��Fe3+���������ɺ�ɫ��
��4���ɵ�ʧ�����غ��ԭ�Ӹ����غ����TiO2�����ʵ������ټ�������������
��5�����������Ʊ���Һ�����У��ձ��е�NH4Fe(SO4)2��Һ�������������������ʵ���Ũ�ȼ�С���ζ�ʱ����NH4Fe(SO4)2��Һ���������
�����ڵζ��յ��ȡ�ζ��ܶ���ʱ�����ӱ�ҺҺ�棬��ȡ��NH4Fe(SO4)2��Һ���ƫС��
��1�������ͼʾ��֪��A�����Ŀ̶��ϴ���С��Ϊ��Ͳ������B�Ŀ̶���С�´�Ϊ�ζ��ܣ��ɰ�Һ�����͵��֪����Ϊ22.80mL������C��0�̶ȣ�Ϊ�¶ȼƣ��ʴ�Ϊ����Ͳ��22.80mL��
��2���������Ϣ��֪TiCl4����ˮ�ⷴӦ����TiO2xH2O���������ᣬ��Ӧ�Ļ�ѧ����ʽΪTiCl4��(2��x)H2O= TiO2��xH2O����4HCl���ʴ�Ϊ��TiCl4��(2��x)H2O= TiO2��xH2O����4HCl��
��3������Ti3+����Һ�еμ�KSCN��Һ��ָʾ������Һ���Ժ�ɫ����NH4Fe(SO4)2����Һ�ζ�ʱ��Fe3+��Ti3+����������ԭ��Ӧ��Ti3+��������������Ti4+��Fe3+����ԭΪFe2+�����ﵽ�ζ��յ�ʱ���ټ���NH4Fe��SO4��2��Һ����Һ��Fe3+���������ɺ�ɫ���յ�������Ǽ������һ��NH4Fe(SO4)2��Һ����Һ��ɺ�ɫ���Ұ���Ӳ��ָ�ԭ����ɫ���ʴ�Ϊ���������һ��NH4Fe(SO4)2��Һ����Һ��ɺ�ɫ���Ұ���Ӳ��ָ�ԭ����ɫ��
��4���ɵ�ʧ�����غ��ԭ�Ӹ����غ��֪��n��Fe3+��=n��Ti3+��=n��TiO2��=0.5mol/L��0.04L=0.02mol����ԭ��Ʒ��TiO2��������Ϊ![]() ��100%��93%���ʴ�Ϊ��93%��
��100%��93%���ʴ�Ϊ��93%��
��5�����������Ʊ���Һ�����У��ձ��е�NH4Fe(SO4)2��Һ�������������������ʵ���Ũ�ȼ�С���ζ�ʱ����NH4Fe(SO4)2��Һ�������������TiO2��������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�����ڵζ��յ��ȡ�ζ��ܶ���ʱ�����ӱ�ҺҺ�棬��ȡ��NH4Fe(SO4)2��Һ���ƫС������TiO2��������ƫ�ͣ��ʴ�Ϊ��ƫ�͡�

 ��У����ϵ�д�
��У����ϵ�д�