��Ŀ����
����˵����ȷ���� �� ��
����25mL��ʽ�ζ��ܿ���ȷ�ų�8��00mL��ϡ���
�ڿ��ø����pH��ֽ�ⶨ��ˮ��pH��
��ʹ������ƿ������Һ������ʱ���ӣ�������Һ��Ũ��ƫ��
�ܼ���������ʹ����ʯ��ˮ����ǵ���ɫ�������ɣ���ԭ��Һ��һ���д���CO ���ڣ�
���ڣ�
����ij�¶��£�����������������Һ��ϣ���Һ�ʼ��ԣ���Һ������Ũ�ȴ�С�Ĺ�ϵһ��Ϊ��c��Na+��>c��CH3COO����>c��OH����>c��H+����
����ͼ�Ƿ�Ӧ��������������ʾ��ͼ������ܷ�����Ӧ���Ȼ�ѧ����ʽ��2A��g��+B��g�� 2C��g�� ��H=QkJ��mol��1��Q<0��
2C��g�� ��H=QkJ��mol��1��Q<0��

����25mL��ʽ�ζ��ܿ���ȷ�ų�8��00mL��ϡ���
�ڿ��ø����pH��ֽ�ⶨ��ˮ��pH��
��ʹ������ƿ������Һ������ʱ���ӣ�������Һ��Ũ��ƫ��
�ܼ���������ʹ����ʯ��ˮ����ǵ���ɫ�������ɣ���ԭ��Һ��һ���д���CO
 ���ڣ�
���ڣ�����ij�¶��£�����������������Һ��ϣ���Һ�ʼ��ԣ���Һ������Ũ�ȴ�С�Ĺ�ϵһ��Ϊ��c��Na+��>c��CH3COO����>c��OH����>c��H+����
����ͼ�Ƿ�Ӧ��������������ʾ��ͼ������ܷ�����Ӧ���Ȼ�ѧ����ʽ��2A��g��+B��g��
 2C��g�� ��H=QkJ��mol��1��Q<0��
2C��g�� ��H=QkJ��mol��1��Q<0��
| A���٢ۢ� | B���ڢܢ� | C���٢ۢ� | D���ۢݢ� |
A
�ڴ�����ˮ�������ԡ�ǿ�����ԡ�Ư���Եȣ�������PH��ֽ�ⶨ��ˮ��PHֵ���ܴ�������������ʹ����ʯ��ˮ����ǵ���ɫ�������ɣ���ԭ��Һ�п�����CO ��HCO3�����ڣ��ݴ�������Ϊ�������ƴ���������c��Na+��>c��OH����>c��CH3COO����>c��H+����
��HCO3�����ڣ��ݴ�������Ϊ�������ƴ���������c��Na+��>c��OH����>c��CH3COO����>c��H+����
 ��HCO3�����ڣ��ݴ�������Ϊ�������ƴ���������c��Na+��>c��OH����>c��CH3COO����>c��H+����
��HCO3�����ڣ��ݴ�������Ϊ�������ƴ���������c��Na+��>c��OH����>c��CH3COO����>c��H+����
��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ
 s) + H2O(g)
s) + H2O(g)  CO(g) + H2(g) ��H�� 0
CO(g) + H2(g) ��H�� 0 
 O 2��g��==H2O��g�� ��H="a" kJ��mol-1��
O 2��g��==H2O��g�� ��H="a" kJ��mol-1�� �ľ���ֵ����ȷ��
�ľ���ֵ����ȷ��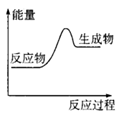
 ���Ȼ�ѧ����ʽ��ȷ���ǣ� ��
���Ȼ�ѧ����ʽ��ȷ���ǣ� ��

 PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ(ͼ�еġ�H��ʾ����1mol���������)��
PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ(ͼ�еġ�H��ʾ����1mol���������)��