��Ŀ����
��t��ʱ��a g NH3��ȫ����ˮ���õ�V mL��Һ���������Һ���ܶ�Ϊd g/cm3����������Ϊ�أ����к�NH4+�����ʵ���Ϊb mol������������ȷ����
- A.���ʵ���������Ϊ�أ���100%
- B.��ˮ�����ʵ���Ũ��Ϊ1000a/(35V)mol��L-1
- C.��Һ��c(OH-)��1000b/V mol��L-1+c(H+)
- D.������Һ���ټ���V mLˮ��������Һ��������������0.5��
C
A����ˮ��Һ����Ϊ����������Һ���ܶ�Ϊ�� g?cm-3�����ΪVmL��������Һ����Ϊ��Vg�����ʰ���������Ϊag�����ʵ���������Ϊag /(��Vg) ��100%����A����
B��ˮ���ܶȱȰ�ˮ���ܶȴ��������İ�ˮ��ˮ��ˮ�������������Ϻ���Һ����������ԭ��ˮ��2������Һ�а�����������ͬ����������������Һ���ʵ���������С��0.5w��
��B����
C����ҺOH-����Դ��һˮ�ϰ���ˮ�ĵ��룬NH4+��Ũ��Ϊbmol /(V��10-3L) ="1000b" /V (mol/L)��һˮ�ϰ�����NH3?H2O?NH4++OH-��һˮ�ϰ��������OH-Ϊ1000b /V (mol/L)��������Һ��OH-Ũ�ȴ���1000b /V (mol/L)����C����
D��a g NH3�����ʵ���Ϊag /(17g/mol) ="a" /17 (mol)����Һ���ΪVmL��������Һ�����ʵ���Ũ��Ϊ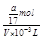 =
=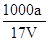 mol/L����D��ȷ��
mol/L����D��ȷ��
��ѡ��D��
�������������ʵ���Ũ�ȡ����������ļ��������ϵ�ȣ��Ѷ��еȣ���������ǽ���Ĺؼ���
A����ˮ��Һ����Ϊ����������Һ���ܶ�Ϊ�� g?cm-3�����ΪVmL��������Һ����Ϊ��Vg�����ʰ���������Ϊag�����ʵ���������Ϊag /(��Vg) ��100%����A����
B��ˮ���ܶȱȰ�ˮ���ܶȴ��������İ�ˮ��ˮ��ˮ�������������Ϻ���Һ����������ԭ��ˮ��2������Һ�а�����������ͬ����������������Һ���ʵ���������С��0.5w��
��B����
C����ҺOH-����Դ��һˮ�ϰ���ˮ�ĵ��룬NH4+��Ũ��Ϊbmol /(V��10-3L) ="1000b" /V (mol/L)��һˮ�ϰ�����NH3?H2O?NH4++OH-��һˮ�ϰ��������OH-Ϊ1000b /V (mol/L)��������Һ��OH-Ũ�ȴ���1000b /V (mol/L)����C����
D��a g NH3�����ʵ���Ϊag /(17g/mol) ="a" /17 (mol)����Һ���ΪVmL��������Һ�����ʵ���Ũ��Ϊ
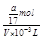 =
=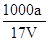 mol/L����D��ȷ��
mol/L����D��ȷ����ѡ��D��
�������������ʵ���Ũ�ȡ����������ļ��������ϵ�ȣ��Ѷ��еȣ���������ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
�����Ŀ
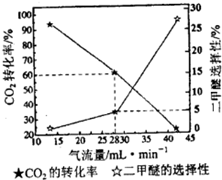
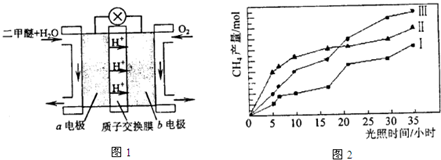
 pD��g��+qE��s������H��0��m��n��p��qΪ��������ȣ���
pD��g��+qE��s������H��0��m��n��p��qΪ��������ȣ���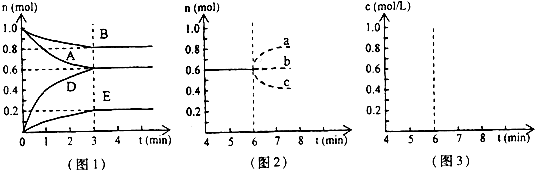

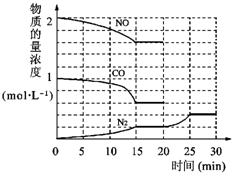 �������ⱸ�������ע���������Լ�����β���ŷŵ�һ����̼��CO�����������NOx������������强��Ϊ������Ⱦ����Ҫ���أ�
�������ⱸ�������ע���������Լ�����β���ŷŵ�һ����̼��CO�����������NOx������������强��Ϊ������Ⱦ����Ҫ���أ� O2+Hb?CO
O2+Hb?CO N2��g��+2CO2��g����H=-113kJ?mol-1
N2��g��+2CO2��g����H=-113kJ?mol-1 H2��g��+CO2��g����ƽ�ⳣ�����¶ȵı仯���±���
H2��g��+CO2��g����ƽ�ⳣ�����¶ȵı仯���±���