��Ŀ����
��2012?������ģ�������ع̶�������CO2����Ч���ٿ����е��������壬���ﻹ���츣���࣮
��1��11km���������������ѧ���������Ժ�����Ȫ���Һ�е�H2S��CO2�ϳɣ�C6H10O5��n��һ�ֵ���ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ
��2��CO2�����ںϳɶ����ѣ�CH3OCH3�����йط�Ӧ���Ȼ�ѧ����ʽΪ��
CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ?mol-1
2CH3OH��g��?H3OCH3��g��+H2O��g����H=-23.5kJ?mol-1
�ٷ�Ӧ2CO2��g��+6H2��g��?CH3OCH3��g��+3H2O��g���ġ�H=
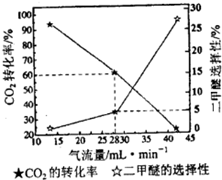
��һ����������CO2��H2�ϳɶ����ѣ���Ӧ����������CO2��ת���ʡ�������ѡ���Ե�Ӱ��������ͼ��ʾ������������Ϊ28mL?min-1������0.3mol��������ͨ��CO2�����ʵ���Ϊ
�۷�Ӧ2CH3OH��g��?CH3OCH3��g��+H2O��g����T��ʱ��ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH��tʱ��ʱ�����c��CH3OH��=0.03mol?L -1��c��CH3OCH3��=0.6mol?L -1����ʱv��
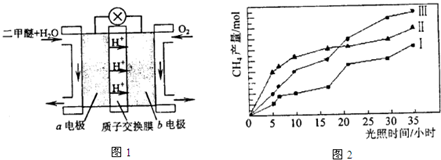
�ܶ�����ȼ�ϵ�صĹ���ԭ����ͼ1��ʾ���õ�ع���ʱ��a�缫�ķ�ӦʽΪ
��3��һ�֡�̼��������Ϊ������CO2�Ĺ�ҵβ��ͨ��NaOH��Һ����������Һ�м�CaO����ַ�Ӧ����ˣ��������·ֽ�õ��ĸ�Ũ��CO2�������Ʊ��״��ȣ��ü��������ѭ��ʹ�õ�����Ϊ
��4�����ù��ܺ�������ɽ�CO2��H2O��g��ת��ΪCH4��O2�����������ʱ��������CO2��H2O��g���ڲ�ͬ�������������£�CH4���������ʱ��ı仯��ͼ2��ʾ����0��30Сʱ�ڣ�CH4�İ����������v����v������v�����Ӵ�С��˳��Ϊ
��1��11km���������������ѧ���������Ժ�����Ȫ���Һ�е�H2S��CO2�ϳɣ�C6H10O5��n��һ�ֵ���ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ
12nH2S+6nCO2=C6H10O5��n+12nS��+7nH2O
12nH2S+6nCO2=C6H10O5��n+12nS��+7nH2O
����2��CO2�����ںϳɶ����ѣ�CH3OCH3�����йط�Ӧ���Ȼ�ѧ����ʽΪ��
CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ?mol-1
2CH3OH��g��?H3OCH3��g��+H2O��g����H=-23.5kJ?mol-1
�ٷ�Ӧ2CO2��g��+6H2��g��?CH3OCH3��g��+3H2O��g���ġ�H=
-121.5kJ/mol
-121.5kJ/mol
����һ����������CO2��H2�ϳɶ����ѣ���Ӧ����������CO2��ת���ʡ�������ѡ���Ե�Ӱ��������ͼ��ʾ������������Ϊ28mL?min-1������0.3mol��������ͨ��CO2�����ʵ���Ϊ
20mol
20mol
��
�۷�Ӧ2CH3OH��g��?CH3OCH3��g��+H2O��g����T��ʱ��ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH��tʱ��ʱ�����c��CH3OH��=0.03mol?L -1��c��CH3OCH3��=0.6mol?L -1����ʱv��
=
=
v��������������������ڡ����ܶ�����ȼ�ϵ�صĹ���ԭ����ͼ1��ʾ���õ�ع���ʱ��a�缫�ķ�ӦʽΪ
CH3OCH3+3H2O-12e-=2CO2+12H+
CH3OCH3+3H2O-12e-=2CO2+12H+
����3��һ�֡�̼��������Ϊ������CO2�Ĺ�ҵβ��ͨ��NaOH��Һ����������Һ�м�CaO����ַ�Ӧ����ˣ��������·ֽ�õ��ĸ�Ũ��CO2�������Ʊ��״��ȣ��ü��������ѭ��ʹ�õ�����Ϊ
�����ƺ���������
�����ƺ���������
����4�����ù��ܺ�������ɽ�CO2��H2O��g��ת��ΪCH4��O2�����������ʱ��������CO2��H2O��g���ڲ�ͬ�������������£�CH4���������ʱ��ı仯��ͼ2��ʾ����0��30Сʱ�ڣ�CH4�İ����������v����v������v�����Ӵ�С��˳��Ϊ
v����v����v����
v����v����v����
��
��������1��������Ŀ��Ϣ��֪H2S��CO2����C6H10O5��n��S������ԭ���غ�����ƽ��
��2���ٸ��ݸ��ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ�����ʵ���ϵ�����мӼ�������Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ��
����Ӧ��ϵ��������Ӧ�����㣻
���ȸ��ݶ����ѵ�ѡ����������������ɵĶ����ѣ�Ȼ��������ʵ���֮�ȵ��ڻ�ѧ������֮�������Ӧ��CO2�����ʵ�����������CO2��ת�������ʵ��ͨ���CO2��
�ۼ���Ũ���̣���ƽ�ⳣ���Ƚϣ����жϷ�Ӧ�Ѿ�ƽ�⣬��v��=v����
�ܷ�Ӧ�����Ƕ����ѵ�ȼ�գ�ԭ��ظ�������������Ӧ���������ڸ����ŵ磬������Ӧ��ԭ��Ӧ�������������ŵ磬��ͼ��֪��a��Ϊ�����������ѷŵ����ɶ�����̼�������ӣ�
��3������Ŀ��Ϣ������ѭ�����õ����ʣ�
��4����ͬʱ���������ʵ����ı仯��Խ����ƽ������Խ����ͬʱ���������ʵ����ı仯��ԽС��ƽ����Ӧ����ԽС��
��2���ٸ��ݸ��ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ�����ʵ���ϵ�����мӼ�������Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ��
����Ӧ��ϵ��������Ӧ�����㣻
���ȸ��ݶ����ѵ�ѡ����������������ɵĶ����ѣ�Ȼ��������ʵ���֮�ȵ��ڻ�ѧ������֮�������Ӧ��CO2�����ʵ�����������CO2��ת�������ʵ��ͨ���CO2��
�ۼ���Ũ���̣���ƽ�ⳣ���Ƚϣ����жϷ�Ӧ�Ѿ�ƽ�⣬��v��=v����
�ܷ�Ӧ�����Ƕ����ѵ�ȼ�գ�ԭ��ظ�������������Ӧ���������ڸ����ŵ磬������Ӧ��ԭ��Ӧ�������������ŵ磬��ͼ��֪��a��Ϊ�����������ѷŵ����ɶ�����̼�������ӣ�
��3������Ŀ��Ϣ������ѭ�����õ����ʣ�
��4����ͬʱ���������ʵ����ı仯��Խ����ƽ������Խ����ͬʱ���������ʵ����ı仯��ԽС��ƽ����Ӧ����ԽС��
����⣺��1��H2S��CO2����C6H10O5��n��S����ѧ����ʽΪ��12nH2S+6nCO2=C6H10O5��n+12nS��+7nH2O��
�ʴ�Ϊ��12nH2S+6nCO2=C6H10O5��n+12nS��+7nH2O��
��2����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ?mol-1 ��
2CH3OH��g��?H3OCH3��g��+H2O��g����H=-23.5kJ?mol-1 ��
�ɸ�˹���ɿ�֪��ͨ���١�2+�ڿɵ�
2CO2��g��+6H2��g��?CH3OCH3��g��+3H2O��g�������H=-49.0kJ/mol��2-23.5kJ/mol=-121.5kJ/mol��
�ʴ�Ϊ��-121.5kJ/mol��
������0.3mol�����ѣ�������Ӧ���ɶ�����%
=6mol����Ӧ�ķ�Ӧ��CO2�����ʵ���Ϊ12mol��ʵ��ͨ���CO2�����ʵ���Ϊ
=20mol��
�ʴ�Ϊ��20mol��
��tʱ��ʱ�����c��CH3OH��=0.03mol?L -1��c��CH3OCH3��=0.6mol?L -1��c��H2O��=0.6mol?L -1��Ũ����Q=
=400=K�����Է�Ӧ�Ѿ�ƽ�⣬��v��=v����
�ʴ�Ϊ��=��
�ܷ�Ӧ�����Ƕ����ѵ�ȼ�գ�ԭ��ظ�������������Ӧ���������ڸ����ŵ磬������Ӧ��ԭ��Ӧ�������������ŵ磮��ͼ��֪��a��Ϊ�����������ѷŵ����ɶ�����̼�������ӣ�a�缫�ĵ缫��ӦʽΪ CH3OCH3-12e-+3H2O=2CO2+12H+��
�ʴ�Ϊ��CH3OCH3+3H2O-12e-=2CO2+12H+��
��3����������Һ�м������ƣ���������ˮ��Ӧ�����������ƣ�̼�������������Ʒ�Ӧ����̼��Ƴ������������ƣ������������Dz����еķ�Ӧ����Կ���ѭ�����ã�̼��Ƹ������������ƺͶ�����̼��������ɵ������ƻ���ѭ�����ã���������ƺ��������ƶ�����ѭ�����ã�
�ʴ�Ϊ�������ƺ��������ƣ�
��4����ͼ2��֪����0��0h�ڣ���������ʵ����仯��Ϊ��n������n������n��������0��30h�ڣ�CH4��ƽ����������v����v����v����
�ʴ�Ϊ��v����v����v����
�ʴ�Ϊ��12nH2S+6nCO2=C6H10O5��n+12nS��+7nH2O��
��2����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ?mol-1 ��
2CH3OH��g��?H3OCH3��g��+H2O��g����H=-23.5kJ?mol-1 ��
�ɸ�˹���ɿ�֪��ͨ���١�2+�ڿɵ�
2CO2��g��+6H2��g��?CH3OCH3��g��+3H2O��g�������H=-49.0kJ/mol��2-23.5kJ/mol=-121.5kJ/mol��
�ʴ�Ϊ��-121.5kJ/mol��
������0.3mol�����ѣ�������Ӧ���ɶ�����%
| 0.3mol |
| 5% |
| 12mol |
| 60% |
�ʴ�Ϊ��20mol��
��tʱ��ʱ�����c��CH3OH��=0.03mol?L -1��c��CH3OCH3��=0.6mol?L -1��c��H2O��=0.6mol?L -1��Ũ����Q=
| 0.6��0.6 |
| 0.032 |
�ʴ�Ϊ��=��
�ܷ�Ӧ�����Ƕ����ѵ�ȼ�գ�ԭ��ظ�������������Ӧ���������ڸ����ŵ磬������Ӧ��ԭ��Ӧ�������������ŵ磮��ͼ��֪��a��Ϊ�����������ѷŵ����ɶ�����̼�������ӣ�a�缫�ĵ缫��ӦʽΪ CH3OCH3-12e-+3H2O=2CO2+12H+��
�ʴ�Ϊ��CH3OCH3+3H2O-12e-=2CO2+12H+��
��3����������Һ�м������ƣ���������ˮ��Ӧ�����������ƣ�̼�������������Ʒ�Ӧ����̼��Ƴ������������ƣ������������Dz����еķ�Ӧ����Կ���ѭ�����ã�̼��Ƹ������������ƺͶ�����̼��������ɵ������ƻ���ѭ�����ã���������ƺ��������ƶ�����ѭ�����ã�
�ʴ�Ϊ�������ƺ��������ƣ�
��4����ͼ2��֪����0��0h�ڣ���������ʵ����仯��Ϊ��n������n������n��������0��30h�ڣ�CH4��ƽ����������v����v����v����
�ʴ�Ϊ��v����v����v����
�����������ǵ��ۺ��⣬�漰����֪ʶ��϶࣬������ѧ���������֪ʶ����������ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ