��Ŀ����
����Ŀ��H2��һ����Ҫ�������Դ��
��1����֪��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H2=-49.0kJmol-1
CH3OH(g)+H2O(g) ��H2=-49.0kJmol-1
CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H3=-41.1kJmol-1
CO2(g)+H2(g) ��H3=-41.1kJmol-1
H2��ԭ CO��Ӧ�ϳɼ״����Ȼ�ѧ����ʽΪ��CO(g)+2H2(g)![]() CH3OH(g) ��H1������H1��___kJmol-1���÷�Ӧ�Է����е�����Ϊ___
CH3OH(g) ��H1������H1��___kJmol-1���÷�Ӧ�Է����е�����Ϊ___
A������ B������ C���κ��¶�������
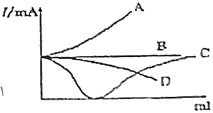
��2�����º�ѹ�£����ݻ��ɱ���ܱ������м��� 1molCO��2.2mol H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)��ʵ����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�ı仯��ͼ��ʾ����P1__P2���жϵ�������_____��
CH3OH(g)��ʵ����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�ı仯��ͼ��ʾ����P1__P2���жϵ�������_____��
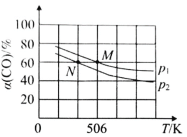
��3������Ӧ CO(g)+2H2(g)![]() CH3OH(g)���¶Ȳ���������㶨Ϊ1���ܱ������з�������Ӧ�����и����ʵ����ʵ�����ʱ��仯�����ʾ��
CH3OH(g)���¶Ȳ���������㶨Ϊ1���ܱ������з�������Ӧ�����и����ʵ����ʵ�����ʱ��仯�����ʾ��
ʱ��/min | 0 | 5 | 10 | 15 |
H2 | 4 | 2 | ||
CO | 2 | 1 | ||
CH3OH(g) | 0 | 0.7 |
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ���־����____(����ĸ)��
A��������ѹǿ���ֲ��� B��2v��
C������������Է����������ֲ��� D�����������ܶȱ��ֲ���
������ʼѹǿΪP0 kPa�����ڸ��¶��·�Ӧ��ƽ�ⳣ��Kp=___(kPa)-2����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�����������
�۷�Ӧ�������õ�λʱ���ڷ�ѹ�ı仯��ʾ����10min��H2�ķ�Ӧ����v(H2)=___kPamin-1��
���𰸡�-90.1 B > ����ӦΪ�����������С�ķ�Ӧ����ѹƽ�����ƣ�CO��ת����������ͼ֪��ͬ�¶�ʱP1��CO��ת���ʴ���P2 AC 9/p02 P0/30
��������
��1�����ݸ�˹��������֪����CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H2=-49.0kJmol-1����CO(g)+H2O(g)
CH3OH(g)+H2O(g) ��H2=-49.0kJmol-1����CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H3=-41.1kJmol-1����+�ڵã�CO(g)+2H2(g)
CO2(g)+H2(g) ��H3=-41.1kJmol-1����+�ڵã�CO(g)+2H2(g)![]() CH3OH(g) ��H1=(-49.0kJmol-1)+( -41.1kJmol-1)= -90.1 kJmol-1,���Դ˷�ӦΪ���ȷ�Ӧ���÷�Ӧ���µ��������Է����У���B��ȷ���𰸣� -90.1�� B��
CH3OH(g) ��H1=(-49.0kJmol-1)+( -41.1kJmol-1)= -90.1 kJmol-1,���Դ˷�ӦΪ���ȷ�Ӧ���÷�Ӧ���µ��������Է����У���B��ȷ���𰸣� -90.1�� B��
��2����ӦCO(g)+2H2(g)![]() CH3OH(g) ������Ӧ�����������С�ķ�Ӧ����������������ʱ������ѹǿ����ѧƽ�������ƶ���CO��ƽ��ת����������ͼʾ��֪��COת����P1>P2������ѹǿ��P1>P2���𰸣�>;����ӦΪ�����������С�ķ�Ӧ����ѹƽ�����ƣ�CO��ת����������ͼ֪��ͬ�¶�ʱP1��CO��ת���ʴ���P2 ��
CH3OH(g) ������Ӧ�����������С�ķ�Ӧ����������������ʱ������ѹǿ����ѧƽ�������ƶ���CO��ƽ��ת����������ͼʾ��֪��COת����P1>P2������ѹǿ��P1>P2���𰸣�>;����ӦΪ�����������С�ķ�Ӧ����ѹƽ�����ƣ�CO��ת����������ͼ֪��ͬ�¶�ʱP1��CO��ת���ʴ���P2 ��
��3����A����Ϊ��Ӧ CO(g)+2H2(g)![]() CH3OH(g)�������������ȵķ�Ӧ�����¶Ȳ���������㶨Ϊ1���ܱ������У���������ѹǿ���ֲ��� ��˵����Ӧ�ﵽƽ���ˣ���A��Ϊ�жϸ÷�Ӧ�ﵽƽ���־����A��ȷ��
CH3OH(g)�������������ȵķ�Ӧ�����¶Ȳ���������㶨Ϊ1���ܱ������У���������ѹǿ���ֲ��� ��˵����Ӧ�ﵽƽ���ˣ���A��Ϊ�жϸ÷�Ӧ�ﵽƽ���־����A��ȷ��
B����v��(H2)=2v��(CH3OH) ʱ������֤����Ӧ�ﵽƽ��״̬�ˣ�����B����Ϊ�жϸ÷�Ӧ�ﵽƽ���־����B����
C������������Է���������ֵ�ϵ���ƽ��Ħ���������͵������������������ʵ�������Ϊ�����ʶ������壬�������������䣬��ѧ��Ӧ�����������ȣ����Ե�ƽ��Ħ���������ֲ��䣬˵����Ӧ�ﵽ��ƽ��״̬����C��ȷ��
D�����������ܶ���=m/V,��Ϊm��V���ֲ��䣬���������䣬����ܶȲ��䣬����Ϊ�жϸ÷�Ӧ�ﵽƽ���־����D����
���Ա���𰸣�AC��
���ݷ�ӦCO(g)+2H2(g)![]() CH3OH(g)�����е����ݿ�֪10minʱ���ﵽƽ�⣬������ʵ���Ϊ��Ϊ��
CH3OH(g)�����е����ݿ�֪10minʱ���ﵽƽ�⣬������ʵ���Ϊ��Ϊ��
CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼ�� 2 4 0
�仯�� 1 2 1
ƽ���� 1 2 1
���ݰ���٤�����ɣ�����ʼѹǿΪP0 kPa����ƽ��ѹǿΪ2/3P0������ƽ�ⳣ��Kp=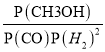 = (2/3P0��1/4)/[2/3P0��1/4��(2/3P0��1/2)2]= 9/p02,�𰸣�9/p02��
= (2/3P0��1/4)/[2/3P0��1/4��(2/3P0��1/2)2]= 9/p02,�𰸣�9/p02��
�۸���ͼ����֪������ʼѹǿΪ2/3P0��10min��ƽ��ѹǿΪ1/3P0����H2�ķ�Ӧ����v(H2)= 1/3P0��10min =1/30 P0kPamin-1,���Ա���𰸣�P0/30��