��Ŀ����
һ����ȡ�����ƵĻ�����������ͼ��ʾ��

��֪��
�ٸ�������Na2S04������Cr2O72-��Fe3+����Fe3+��Cr3+ǡ����ȫ������c��1.0��10-5mol/L��ʱpH�ֱ�Ϊ3.6 ��5��
(1)����AΪ_______(�ѧʽ)��
(2)������ͼ�ܽ��(S)-�¶�(T)���ߣ�����B����ѷ���Ϊ_________(����ĸ���)��

a������Ũ�������ȹ��� b������Ũ�������½ᾧ������
(3)�ữ��Cr2O72-�ɱ�SO32-��ԭ��Cr3+�����ӷ���ʽΪ____________����CΪ______________��Cr(OH)3���ܶȻ�����Ksp[Cr(OH)3]=______________��
(4)���ݷ�Ӧ2CrO42-+2H+ Cr2O72-+H2O ����ͼ��ʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ��____________������缫��ӦʽΪ____________��ͨ��2mol���ӣ�����Cr2O72-�����ʵ�����__________________��
Cr2O72-+H2O ����ͼ��ʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ��____________������缫��ӦʽΪ____________��ͨ��2mol���ӣ�����Cr2O72-�����ʵ�����__________________��

��ϰ��ϵ�д�
�����Ŀ





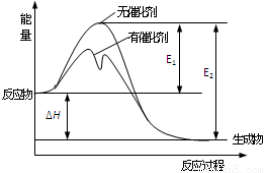
 2SO3(g)��Ӧ�����������仯��ͼ��ʾ��ͼ��E1��ʾ����Ӧ�Ļ�ܣ�E2��ʾ�淴Ӧ�Ļ�ܣ��������й�������ȷ����
2SO3(g)��Ӧ�����������仯��ͼ��ʾ��ͼ��E1��ʾ����Ӧ�Ļ�ܣ�E2��ʾ�淴Ӧ�Ļ�ܣ��������й�������ȷ����