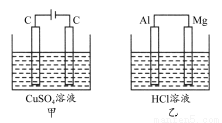
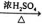̀âÄ¿ÄÚÈƯ
ij¿ÎÍâ»î¶¯Đ¡×éÓĂÈçͼװÖĂ½øĐĐʵÑ飬ÊԻشđÏÂÁĐÎỀâ¡£
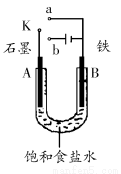
(1)Èô¿ªÊ¼Ê±¿ª¹ØKÓëaÁ¬½Ó£¬ỘB¼«µÄµç¼«·´Ó¦Ê½Îª________________¡£
(2)Èô¿ªÊ¼Ê±¿ª¹ØKÓëbÁ¬½Ó£¬ỘB¼«µÄµç¼«·´Ó¦Ê½Îª________£¬×Ü·´Ó¦µÄÀë×Ó·½³̀ʽΪ__________________________¡£
ÓĐ¹ØÉÏÊöʵÑ飬ÏÂÁĐ˵·¨ƠưÈ·µÄÊÇ(̀îĐ̣ºÅ)________________¡£
¢ÙÈÜ̉ºÖĐNa£«Ị̈A¼«̉ƶ¯¡¡¢Ú´ÓA¼«´¦̉Ư³öµÄÆø̀åÄÜʹʪÈóµÄKIµí·ÛÊÔÖ½±äÀ¶¡¡¢Û·´Ó¦̉»¶Îʱ¼äºó¼ÓÊÊÁ¿ÑÎËá¿É»Ö¸´µ½µç½âÇ°µç½âÖʵÄŨ¶È¡¡¢ÜÈô±ê×¼×´¿öÏÂB¼«²úÉú2.24LÆø̀壬ỘÈÜ̉ºÖĐת̉Æ0.2molµç×Ó
(3)¸ĂĐ¡×éͬѧÈÏΪ£¬Èç¹ûÄ£Ä⹤̉µÉÏÀë×Ó½»»»Ä¤·¨ÖÆÉƠ¼îµÄ·½·¨£¬ÄÇĂ´¿É̉ÔÉèÏëÓĂÈçͼװÖõç½âẠ́Ëá¼ØÈÜ̉ºÀ´ÖÆÈ¡ÇâÆø¡¢ÑơÆø¡¢Ạ́ËáºÍÇâÑơ»¯¼Ø¡£
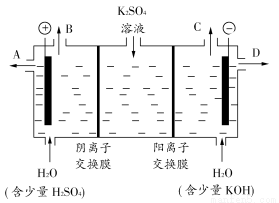
¢Ù¸Ăµç½â²ÛµÄÑô¼«·´Ó¦Ê½Îª________________¡£´Ëʱͨ¹ửơÀë×Ó½»»»Ä¤µÄÀë×ÓÊư________(̀î¡°´óÓÚ¡±¡¢¡°Đ¡ÓÚ¡±»̣¡°µÈÓÚ¡±)ͨ¹ưÑôÀë×Ó½»»»Ä¤µÄÀë×ÓÊư¡£
¢ÚÖÆµĂµÄÇâÑơ»¯¼ØÈÜ̉º´Ó³ö¿Ú(̀î¡°A¡±¡¢¡°B¡±¡¢¡°C¡±»̣¡°D¡±)________µ¼³ö¡£
¢Ûͨµç¿ªÊ¼ºó£¬̉ơ¼«¸½½üÈÜ̉ºpH»áÔö´ó£¬Çë¼̣ÊöỘ̉
______________________________________________________________¡£
¢ÜÈô½«ÖÆµĂµÄÇâÆø¡¢ÑơÆøºÍÇâÑơ»¯¼ØÈÜ̉º×éºÏΪÇâÑơȼÁϵç³Ø£¬Ộµç³ØƠư¼«µÄµç¼«·´Ó¦Ê½Îª________________¡£
(1)Fe£2e£=Fe2£«
(2)2H£«£«2e£=H2¡ü¡¡2Cl££«2H2O 2OH££«H2¡ü£«Cl2¡ü¡¡¢Ú
2OH££«H2¡ü£«Cl2¡ü¡¡¢Ú
(3)¢Ù4OH££4e£=2H2O£«O2¡ü¡¡Đ¡ÓÚ
¢ÚD
¢ÛH£«ÔÚ̉ơ¼«¸½½ü·Åµç£¬̉ưÆđË®µÄµçÀëƽºâỊ̈Ó̉̉ƶ¯£¬Ê¹c(OH£)>c(H£«)
¢ÜO2£«2H2O£«4e£=4OH£
¡¾½âÎö¡¿(1)¿ª¹ØKÓëaÏàÁ¬£¬×°ÖĂ¹¹³ÉÔµç³Ø£¬Feʧȥµç×ÓΪԵç³Ø¸º¼«¡£(2)¿ª¹ØKÓëbÏàÁ¬£¬×°ÖĂ¹¹³Éµç½âNaClÈÜ̉ºµÄµç½â³Ø£¬BΪµç½â³ØµÄ̉ơ¼«£¬ÈÜ̉ºÖеÄH£«ÔÚB¼«·ÅµçÉú³ÉH2¡£µç½â¹ư³̀ÖĐNa£«Ó¦¸ĂỊ̈̉ơ¼«B̉ƶ¯£»A¼«²úÉúµÄÆø̀åΪCl2£¬Cl2Äܽ«I£Ñơ»¯ÎªI2£¬I2Óöµí·Û±äÀ¶£¬¸ù¾Ưµç½â·´Ó¦£º2NaCl£«2H2O 2NaOH£«H2¡ü£«Cl2¡ü£¬µç½ẩ»¶Îʱ¼äºóÈô¼ÓÈëÑÎËá»áÔö¼ÓH2OµÄÖÊÁ¿£¬Ó¦Í¨ÈëHClÆø̀åʹÆä»Ö¸´µ½µç½âÇ°µç½âÖʵÄŨ¶È£»Èô±ê×¼×´¿öÏÂB¼«²úÉú2.24LÆø̀壬¼´0.1mol H2£¬ỘÓĐ0.2molµç×Ó·¢Éúת̉Æ£¬µ«ÔÚÈÜ̉ºÖĐת̉ƵIJ»Êǵç×Ó£¬¶øÊÇÀë×Ó¡£(3)¢ÙÈÜ̉ºÖеÄOH£ÔÚÑô¼«Ê§µç×Ó²úÉúO2£º4OH££4e£=2H2O£«O2¡ü£¬Ëù̉ÔÔÚB¿Ú·Å³öO2£¬´ÓA¿Úµ¼³öH2SO4¡£ÈÜ̉ºÖеÄH£«ÔÚ̉ơ¼«µĂµ½µç×Ó²úÉúH2£º2H£«£«2e£=H2¡ü£¬Ộ´ÓC¿Ú·Å³öH2£¬´ÓD¿Úµ¼³öKOHÈÜ̉º¡£̣̉SO42¡ªËù´øµçºÉÊư´óÓÚK£«Ëù´øµçºÉÊư£¬SO42¡ªÍ¨¹ửơÀë×Ó½»»»Ä¤£¬K£«Í¨¹ưÑôÀë×Ó½»»»Ä¤£¬Ëù̉Ôͨ¹ưÑôÀë×Ó½»»»Ä¤µÄÀë×ÓÊư´óÓÚͨ¹ửơÀë×Ó½»»»Ä¤µÄÀë×ÓÊư¡£O2¡¢H2¡¢KOHÈÜ̉º¹¹³ÉȼÁϵç³Øʱ£¬O2ÔÚµç³ØƠư¼«·Åµç£ºO2£«4e££«2H2O=4OH£¡£
2NaOH£«H2¡ü£«Cl2¡ü£¬µç½ẩ»¶Îʱ¼äºóÈô¼ÓÈëÑÎËá»áÔö¼ÓH2OµÄÖÊÁ¿£¬Ó¦Í¨ÈëHClÆø̀åʹÆä»Ö¸´µ½µç½âÇ°µç½âÖʵÄŨ¶È£»Èô±ê×¼×´¿öÏÂB¼«²úÉú2.24LÆø̀壬¼´0.1mol H2£¬ỘÓĐ0.2molµç×Ó·¢Éúת̉Æ£¬µ«ÔÚÈÜ̉ºÖĐת̉ƵIJ»Êǵç×Ó£¬¶øÊÇÀë×Ó¡£(3)¢ÙÈÜ̉ºÖеÄOH£ÔÚÑô¼«Ê§µç×Ó²úÉúO2£º4OH££4e£=2H2O£«O2¡ü£¬Ëù̉ÔÔÚB¿Ú·Å³öO2£¬´ÓA¿Úµ¼³öH2SO4¡£ÈÜ̉ºÖеÄH£«ÔÚ̉ơ¼«µĂµ½µç×Ó²úÉúH2£º2H£«£«2e£=H2¡ü£¬Ộ´ÓC¿Ú·Å³öH2£¬´ÓD¿Úµ¼³öKOHÈÜ̉º¡£̣̉SO42¡ªËù´øµçºÉÊư´óÓÚK£«Ëù´øµçºÉÊư£¬SO42¡ªÍ¨¹ửơÀë×Ó½»»»Ä¤£¬K£«Í¨¹ưÑôÀë×Ó½»»»Ä¤£¬Ëù̉Ôͨ¹ưÑôÀë×Ó½»»»Ä¤µÄÀë×ÓÊư´óÓÚͨ¹ửơÀë×Ó½»»»Ä¤µÄÀë×ÓÊư¡£O2¡¢H2¡¢KOHÈÜ̉º¹¹³ÉȼÁϵç³Øʱ£¬O2ÔÚµç³ØƠư¼«·Åµç£ºO2£«4e££«2H2O=4OH£¡£

 ÔĶÁ¿́³µÏµÁĐ´đ°¸
ÔĶÁ¿́³µÏµÁĐ´đ°¸