��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ����,�������е���ĸ�ֱ����ijһ��ѧԪ�ء�
A | ||||||||
B | C | |||||||
D | E | F | ||||||
�ش��������⣺
(1)Ԫ��B��Ԫ�ط���Ϊ______������Ԫ�����ڱ��е�λ����__________��
(2)D�ļ����ӵĽṹʾ��ͼΪ_________����������������Ӧˮ�����к��еĻ�ѧ����_____________��D������ˮ��Ӧ�Ļ�ѧ����ʽΪ____________________��
(3)�õ���ʽ��ʾEF2���γɹ���_________________________________��
(4)��A2��C2���ɼ���ȼ�ϵ�أ���õ�صĸ�����Ӧ����ʽΪ_______________���õ�ع���������ÿͨ��2mol��������Ҫ��C2���Ϊ___________(��״��)��
(5)C��D�ڸ����������γɻ�����Ļ�ѧʽΪ_________���������̼��Ӧ�Ļ�ѧ����ʽΪ_________________________��
���𰸡� N �ڶ����ڵڢ�A��  ���Ӽ���(����)���ۼ� 2Na+2H2O
���Ӽ���(����)���ۼ� 2Na+2H2O![]() 2NaOH+ H2��
2NaOH+ H2�� ![]() H2 �C 2e��+ 2OH��
H2 �C 2e��+ 2OH��![]() 2H2O 11.2L Na2O2 2Na2O2 +2CO2
2H2O 11.2L Na2O2 2Na2O2 +2CO2![]() 2Na2CO3+O2
2Na2CO3+O2
������������Ԫ�������ڱ��е�λ�ÿ�֪��AΪH��BΪN��CΪO��DΪNa��EΪMg��FΪCl��
(1) BΪN����Ԫ�����ڱ���λ�ڵڶ����ڵڢ�A�壬�ʴ�Ϊ��N���ڶ����ڵڢ�A����
(2) DΪNa�������ӵĽṹʾ��ͼΪ �����������������ӻ�������к��еĻ�ѧ�������Ӽ���(����)���ۼ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+ H2�����ʴ�Ϊ��
�����������������ӻ�������к��еĻ�ѧ�������Ӽ���(����)���ۼ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+ H2�����ʴ�Ϊ�� �����Ӽ���(����)���ۼ���2Na+2H2O=2NaOH+ H2����
�����Ӽ���(����)���ۼ���2Na+2H2O=2NaOH+ H2����
(3)�õ���ʽ��ʾMgCl2���γɹ���Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(4)��H2��O2���ɼ���ȼ�ϵ�أ�ͨ��������Ϊ������������Ӧ����ʽΪH2 �C 2e��+ 2OH��= 2H2O���õ�ع���������ÿͨ��2mol��������Ҫ��O2�����ʵ���Ϊ![]() =0.5mol���ڱ�״���µ����Ϊ0.5mol��22.4L/mol=11.2L���ʴ�Ϊ��H2 �C 2e��+ 2OH��= 2H2O�� 11.2L��
=0.5mol���ڱ�״���µ����Ϊ0.5mol��22.4L/mol=11.2L���ʴ�Ϊ��H2 �C 2e��+ 2OH��= 2H2O�� 11.2L��
(5)���������ڸ��������·�Ӧ���ɹ������������������������̼��Ӧ�Ļ�ѧ����ʽΪ2Na2O2 +2CO2= 2Na2CO3+O2���ʴ�Ϊ��Na2O2��2Na2O2 +2CO2= 2Na2CO3+O2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��NH3��һ����Ҫ�Ļ���ԭ�ϣ�����������������;�㷺��
��1����֪��
���ۼ� | ����/ kJ��mol-1 |
H�DH | 436 |
N��N | 946 |
N�DH | 391 |
ע������̬������1 molij�ֹ��ۼ���Ҫ���յ����������Ǹù��ۼ��ļ��ܡ�
N2 (g)��3 H2 (g)![]() 2 NH3 (g) H =____kJ��mol-1
2 NH3 (g) H =____kJ��mol-1
��2��һ���¶��£�����ݵ��ܱ������г���N2��H2������Ӧ��N2 ��3H2 ![]() 2NH3����ø����Ũ����ʱ��仯��ͼ1��ʾ��
2NH3����ø����Ũ����ʱ��仯��ͼ1��ʾ��
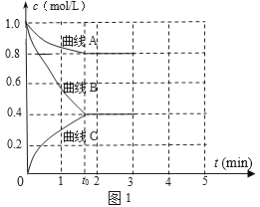
�ٱ�ʾc(N2)��������__�������A����������B��������C������
��0��t0ʱ��H2��ʾ��Ӧ����v(H2)____mol��L-1��min-1��
��������˵���÷�Ӧ�ﵽƽ�����____��
a����������ѹǿ���ٱ仯
b��2c(H2)= 3c(NH3)
c�����������������ٱ仯
d��NH3������������ٱ仯
��3��DZͧ��ʹ�õ�Һ��-Һ��ȼ�ϵ�ع���ԭ����ͼ2��ʾ��
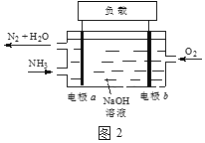
�ٵ缫b������____��
�ڵ������Һ��OH-������____�ƶ�����缫a���缫b������
�۵缫a�ĵ缫��ӦʽΪ____��
��4����ͨ��NH3��NaClO��Ӧ���Ƶû��ȼ���£�N2H4�����÷�Ӧ�Ļ�ѧ��Ӧ����ʽ��____��