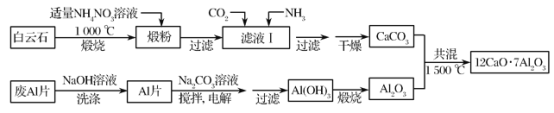
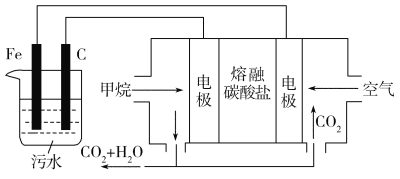
��Ŀ����
����Ŀ��(1)��ǰ�����������ѳ�Ϊ��Ҫ���������̡�
��2014��1�£��������涨��ѧУ����������ֹ���̡������й�˵���в���ȷ����_____(����ĸ���ţ���ͬ����
A.���̻�����ں������������Ⱦ
B.���뽹�͡���Ŷ���������ɵ��¶��ֲ���
C.N2�� CO2��CO����Ŷ����������ڿ�����Ⱦ��
�������������ܻ���ء�����������______��
A.�������սո�
B.������β��ϵͳװ�ô�ת����
C.��չú��������Һ���Ƚྻú����
�����д��������ķ����У�����ȷ����____��
A.�������÷�ֽ
B.��������������
C.����Ͼɵ��
������������ѭ����������������Դ����ԭ�����������ռ���ʵ������ԭ��Ĵ�ʩ֮����������ͼ��ʾ����ʾΪ________
![]()
A.�ɻ����� B.�к����� C.��������
���ҹ����з����ġ����������ձ����У��������ʲ�������Ҫ��Ⱦ�����____��
A.�������� B.������̼ C.�������� D.�����������
(2)������������������ͷ�չ����Ҫ���ʻ�����
���Թܡ��ջ�����ƿ�Ȼ�ѧ��������Ҫ������____ (����ĸ���ţ���
A.���� B. �մ�
�ڸ�����Ŀǰ���������Ͻ𡣸����Ӵ���ˮ�����绯ѧ��ʴ���为����ӦʽΪ______����ˣ�Ϊ��ֹ�ִ����屻��ʴ�����ڴ����ϰ�װһ������______���п���ɡ�ͭ������
���𰸡�C A C A B A Fe-2e-=Fe2+ п
��������
��1)��A.��������������ɿ����������������������̻�Ӱ�컷��������Ӱ���������彡����Ҳ�����ε����˺��������������̣�A��������⣻B.�̲��к�����Ŷ���һ����̼���к����壬���շ���״�������Σ������ļ�ȱѪ���շ�����ʧ���ȣ�B��������⣻C.������̼�͵��������ڿ�����Ⱦ�C��������⣬����ѡC��
��A.�������սոѻ���������̳����Ӷ�����������A��������⣻B. ������β��ϵͳװ�ô�ת�������Լ�������β���Լ��̳����ŷţ����������ķ�����B����������⣻C.ú��������Һ���������Դ���������Լ��ٶ���������к�������ŷţ������̳������������ķ�����C��������⣬����ѡA��
��A.����ֽ�ռ������������ۺ����ã����Ϊ����A��������⣻B.���������ޣ����۴ӽ�Լ��Դ�����Ǵӱ������������ƻ����ȷ��涼���ش����壬B��������⣻C.�ϵ���к����ؽ�����������������Ҫ�������ã�C��������⣬����ѡC��
��![]() �����еļ�ͷ������ͬ�������ɻ������ã�����ѡA��
�����еļ�ͷ������ͬ�������ɻ������ã�����ѡA��
�ݿ��������ձ��еĿ�����Ⱦ���У�һ����̼���������������������������������̼�����ڿ�����Ⱦ�����ѡB��
(2) ���Թܡ��ջ�����ƿ�Ȼ�ѧ�������ڲ�������������ѡA��
�ڸ�����ʧȥ���ӣ���������ʽΪ��Fe-2e-=Fe2+���������壬���Բ�ȡ����������������������ͨ���ͽϻ��ý����������Ӷ��������壬��˿��ڴ����ϰ�װһ������п����Ϊ��Fe-2e-=Fe2+��п��

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�����Ŀ������ΪԪ�����ڱ��е�һ���֣��û�ѧʽ��Ԫ�ط��Żش��������⡣
�� ���� | IA | ��A | ��A | ��A | VA | ��A | ��A | 0 |
�� |
|
| ||||||
�� |
|
|
|
|
|
| ||
�� |
��1��9��Ԫ���У���ѧ��������õ���______��ԭ�ӽṹʾ��ͼΪ____________��
��2����ЩԪ���У�����������ˮ�����м�����ǿ�ļ���__________��д��Ԫ��![]() �ĵ������䷴Ӧ�����ӷ���ʽ��___________________________________��
�ĵ������䷴Ӧ�����ӷ���ʽ��___________________________________��
��3������������������������Ԫ����______ ��д��������������NaOH��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽ�� ____________________________________ ��
��4���õ���ʽ��ʾԪ��![]() ��
��![]() ��ɵĻ�������γɹ��� _____________________________���û��������� ______ ����������������������
��ɵĻ�������γɹ��� _____________________________���û��������� ______ ����������������������![]() �����
�����