��Ŀ����
С�մ�θ��ƽ����ϲ���dz��õ��к�θ���ҩ�
��1��С�մ�ÿƬ��0��50gNaHCO3,2ƬС�մ�Ƭ��θ����ȫ�кͣ����к͵�H+�� mol
��2��θ��ƽÿƬ��0��245gAl��OH��3���к�θ��ʱ��6ƬС�մ��൱��θ��ƽ Ƭ
��3����ϲ�Ļ�ѧ�ɷ�������þ�ļ�ʽ�Σ�
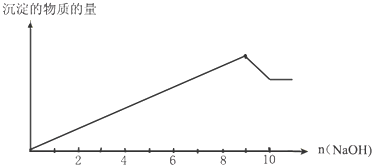
ȡ�ü�ʽ��3��01g,����2��0mol/L����ʹ���ܽ⣬����������42��5mLʱ��ʼ����CO2������������45��0mLʱ������ȫ��Ӧ������ü�ʽ����Ʒ����������̼��������ʵ���֮��
��1��0.012mol ��2��3.8 (3)16��1
����������1��2ƬС�մ�Ƭ�к���̼��������1.0g�����Ը��ݷ���ʽHCO3����H��=H2O��CO2����֪�����к͵���������2��0.5/(23+1+12+16��3)=0.012mol��
��2��6ƬС�մ����к���������0.012mol��3��0.036mol������ݷ���ʽAl(OH)3��3H��=Al3����3H2O��֪����������������0.012mol��������0.012mol��78g/mol��0.936g������0.936��0.245��3.8��
��3��42��5mL��45��0mL��������ʵ����ֱ���0.085mol��0.09mol������ݷ���ʽOH����H��=H2O��H����CO32��=HCO3����HCO3����H��=H2O��CO2����֪��HCO3�������ʵ�����0.005mol������CO32����Ӧ����������0.005mol�����Ժ�OH����Ӧ����������0.08mol����ʽ����Ʒ����������̼��������ʵ���֮��0.08�U0.005��16�U1��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�