��Ŀ����
С�մ�θ��ƽ����ϲ���dz��õ��к�θ���ҩ�
��1��С�մ�ƬÿƬ��0.42gNaHCO3��θ��ƽÿƬ��0.26gAl��OH��3���к�θ��ʱ��3ƬС�մ�Ƭ�൱��θ��ƽ Ƭ��
��2����ϲ����̼��þ��Ƭ����ѧʽΪAlxMgy��OH��zCO3?nH2O����Է�������602��
��ȡ�ü�ʽ��3.01g������1.00mol?L-1����ʹ���ܽ⣬����������85.0mLʱ��ʼ����CO2������������90.0mLʱ���÷�Ӧ��ȫ������ü�ʽ����Ʒ����������̼��������ʵ���֮���� ��
����������ʽ��������������Һ�м�������������ƣ����������������ʵ����͵õ����������ʵ����Ĺ�ϵ��ͼ��ʾ�����ϲ�Ļ�ѧʽ�� ��
��1��С�մ�ƬÿƬ��0.42gNaHCO3��θ��ƽÿƬ��0.26gAl��OH��3���к�θ��ʱ��3ƬС�մ�Ƭ�൱��θ��ƽ
��2����ϲ����̼��þ��Ƭ����ѧʽΪAlxMgy��OH��zCO3?nH2O����Է�������602��
��ȡ�ü�ʽ��3.01g������1.00mol?L-1����ʹ���ܽ⣬����������85.0mLʱ��ʼ����CO2������������90.0mLʱ���÷�Ӧ��ȫ������ü�ʽ����Ʒ����������̼��������ʵ���֮����
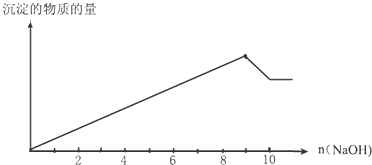
����������ʽ��������������Һ�м�������������ƣ����������������ʵ����͵õ����������ʵ����Ĺ�ϵ��ͼ��ʾ�����ϲ�Ļ�ѧʽ��

��������1�����ݷ���ʽ�ҳ�����������̼������֮��Ĺ�ϵ��
��2����ʽ̼���������ᷴӦ���������кͼ�ٺ�̼��������̼�����Σ�Ȼ��Ż�ų�CO2���壬�漰���Ļ�ѧ��Ӧ�У�
��OH-+H+=H2O��
��CO32-+H+�THCO3-��
��HCO3-+H+�TH2O+CO2����
���������غ㣬��ϻ�ѧ����ʽ���㣮
��2����ʽ̼���������ᷴӦ���������кͼ�ٺ�̼��������̼�����Σ�Ȼ��Ż�ų�CO2���壬�漰���Ļ�ѧ��Ӧ�У�
��OH-+H+=H2O��
��CO32-+H+�THCO3-��
��HCO3-+H+�TH2O+CO2����
���������غ㣬��ϻ�ѧ����ʽ���㣮
����⣺��1�����൱������������Ƭ��Ϊy
�ɷ���ʽAl��OH��3+3HCl=AlCl3+3H2O��NaHCO3+HCl�TNaCl+H2O+CO2��
�ù�ϵʽAl��OH��3��3HCl��3NaHCO3
78 3��84
0.26g��y 0.42g��3
=
y=1.5Ƭ
�ʴ�Ϊ��1.5��
��2���ٸü�ʽ��3.01g�����ʵ���Ϊ
=0.005mol��
����������85mLʱ����ʼ����CO2������������90.0mLʱ���÷�Ӧ��ȫ��
�������ķ�Ӧ�ֱ�Ϊ����OH-+H+=H2O��
��CO32-+H+�THCO3-��
��HCO3-+H+�TH2O+CO2����
�ɵ�n��CO2��=n��CO32-��=5��10-3L��1mol/L=0.005mol��
���к�OH-��Ҫ����85ml-5ml=80mL������n��OH-��=80��10-3L��1mol/L=0.08mol��
��������̼��������ʵ���֮��0.08mol��0.005mol=16��1��
�ʴ�Ϊ��n��OH-����n��CO32-��=16��1��
����ͼ֪�������ӵ����ʵ���Ϊ1���ܽ������������Ϊ3��þ���ӵ����ʵ���Ϊ3��
n��Al3+����n��Mg2+��=1��3
���ݵ���غ㣬3n��Al3+��+2n��Mg2+��=n��OH-��+2n��CO32-��=0.08mol+2��0.005mol
���n��Al3+��=0.01mol
n��Mg2+��=0.03mol
���������غ㣬�����к��нᾧˮ��
3.01g-0.005mol��60g/mol-0.08mol��17g/mol-0.03mol��24g/mol-0.01mol��27g/mol=0.36g��
���ʵ���Ϊ
=0.02mol��
�ۺ����ϣ��ü�ʽ̼���εĻ�ѧʽΪAl2Mg6��OH��16CO3?4H2O��
�ʴ�Ϊ��Al2Mg6��OH��16CO3?4H2O��
�ɷ���ʽAl��OH��3+3HCl=AlCl3+3H2O��NaHCO3+HCl�TNaCl+H2O+CO2��
�ù�ϵʽAl��OH��3��3HCl��3NaHCO3
78 3��84
0.26g��y 0.42g��3
| 78 |
| 0.26g��y |
| 3��84 |
| 0.42g��3 |
y=1.5Ƭ
�ʴ�Ϊ��1.5��
��2���ٸü�ʽ��3.01g�����ʵ���Ϊ
| 3.01 |
| 602 |
����������85mLʱ����ʼ����CO2������������90.0mLʱ���÷�Ӧ��ȫ��
�������ķ�Ӧ�ֱ�Ϊ����OH-+H+=H2O��
��CO32-+H+�THCO3-��
��HCO3-+H+�TH2O+CO2����
�ɵ�n��CO2��=n��CO32-��=5��10-3L��1mol/L=0.005mol��
���к�OH-��Ҫ����85ml-5ml=80mL������n��OH-��=80��10-3L��1mol/L=0.08mol��
��������̼��������ʵ���֮��0.08mol��0.005mol=16��1��
�ʴ�Ϊ��n��OH-����n��CO32-��=16��1��
����ͼ֪�������ӵ����ʵ���Ϊ1���ܽ������������Ϊ3��þ���ӵ����ʵ���Ϊ3��
n��Al3+����n��Mg2+��=1��3
���ݵ���غ㣬3n��Al3+��+2n��Mg2+��=n��OH-��+2n��CO32-��=0.08mol+2��0.005mol
���n��Al3+��=0.01mol
n��Mg2+��=0.03mol
���������غ㣬�����к��нᾧˮ��
3.01g-0.005mol��60g/mol-0.08mol��17g/mol-0.03mol��24g/mol-0.01mol��27g/mol=0.36g��
���ʵ���Ϊ
| 0.36g |
| 18g/mol |
�ۺ����ϣ��ü�ʽ̼���εĻ�ѧʽΪAl2Mg6��OH��16CO3?4H2O��
�ʴ�Ϊ��Al2Mg6��OH��16CO3?4H2O��
���������⿼�����ʵ����ļ��㣬��Ŀ�Ѷ��еȣ�ע���ʽ̼���������ᷴӦ���������кͼ�ٺ�̼��������̼�����Σ�Ȼ��Ż�ų�CO2���壬������Ըù��̣��ͻ�ʹ������ྶͥ��

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ