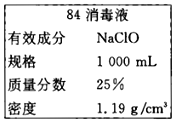
��Ŀ����
����Ŀ��ijʵ��С��̽��H2O2�ֽ�����ʼ�Ӱ�����أ�����ͬ�¶��°����±���ʾ�ķ������ʵ�顣

��1������ʵ�鷽���У�̽���ı�������ֻ�д�����ʵ�������_____��_____��
��2��ʵ��ܹ������������������ʺ�ʱ������ƹ�ϵ��ͼ��ʾ���жϸ÷�Ӧ�Ƿ�Ӧ������������������������______��
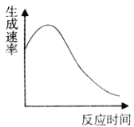
��3��ʵ��ݣ����Թ��м���10mL 0.4mol/L H2O2��Һ����������������һ���¶��²�ò�ͬʱ������O2�������������Ϊ��״�������±���ʾ�����跴Ӧ������Һ��������ֲ��䣩��
![]()
�ٷ�Ӧ6min��H2O2�ֽ���_______%��
��0��6min����H2O2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ��(H2O2)=________molL-1min-1��
���𰸡��� �� ���� 50 0.03
��������
��1��̽���ı�������ֻ�д�����ʵ�������Ҫѡ�д�����ʵ�飬��ܷ������⣬ͬʱΪ�˱�֤����Ψһ����Ҫѡ�������գ��ʺ�����ʵ�����Ǣۢܣ�
��2���տ�ʼʱ����Ӧ���������ӣ�����Ϊ��Ӧ���ȣ��¶����ӿ췴Ӧ���ʣ�������Ӧ������С������ΪH2O2��Ũ�ȼ�������ģ��ʿ����жϳ��÷�Ӧ�Ƿ��ȷ�Ӧ��
��3���ٷ�Ӧ6min�ڣ�����O2 22.4mL����0.001mol������ݷ�Ӧ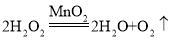 �ɼ�����ѷֽ��H2O2Ϊ0.002mol����H2O2�ķֽ���Ϊ
�ɼ�����ѷֽ��H2O2Ϊ0.002mol����H2O2�ķֽ���Ϊ![]() =50%��
=50%��
����(H2O2)=![]() =
=![]() =0.03molL-1min-1��
=0.03molL-1min-1��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�