��Ŀ����
��14�֣�I����֪��C(s)��H2O(g) CO(g)��H2(g) ��H
CO(g)��H2(g) ��H
һ���¶��£���1.0 L�ܱ������з���1 mol C��s����1 mol H2O(g)���з�Ӧ,��Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±���
�ش��������⣺
��1��������Щѡ�����˵���ÿ��淴Ӧ�Ѵ�ƽ��״̬ ��
A�����������ܶȲ��ٷ����ı� B������1 mol H2O��g����ͬʱ����1 mol H2
C����H���� D��v��(CO) = v��(H2)
��2������ѹǿP����ʼѹǿP0��ʾ��Ӧ��ϵ�������ʵ���n����n����____ mol���ɱ������ݼ��㷴Ӧ��ƽ��ʱ����Ӧ��H2O(g)��ת���ʦ� =_____����ȷ��С�����ڶ�λ����
�����ʼ��仯�����ڹ�ũҵ������������Ҫ��Ӧ�á�
��1����֪25��ʱ��xSO2 (g)��2xCO(g)��2xCO2 (g)��Sx (s) ��H��ax kJ/mol ��
2xCOS(g)��xSO2 (g)��2xCO2 (g)��3Sx (s) ��H��bx kJ/mol�� ��
��ӦCOS(g)����CO(g)��Sx (s)���Ȼ�ѧ����ʽ�� ��
��2��������ʵ���Ũ��Na2S��NaOH�����Һ�еμ�ϡ����������������H2S��HS?��S2?�ķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������μ���������Ĺ�ϵ��ͼ��ʾ�����Եμӹ���H2S������ݳ������Է�����
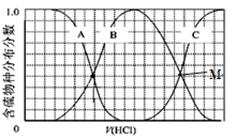
��B���ߴ��� �����仯(�������ű�ʾ)���μӹ����У���Һ��һ��������
c(Na+)= ��
��M�㣬��Һ����Ҫ�漰�����ӷ���ʽ ��
 CO(g)��H2(g) ��H
CO(g)��H2(g) ��Hһ���¶��£���1.0 L�ܱ������з���1 mol C��s����1 mol H2O(g)���з�Ӧ,��Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±���
| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
| ��ѹǿp/100 kPa | 4.56 | 5.14 | 5.87 | 6.30 | 7.24 | 8.16 | 8.18 | 8.20 | 8.20 |
��1��������Щѡ�����˵���ÿ��淴Ӧ�Ѵ�ƽ��״̬ ��
A�����������ܶȲ��ٷ����ı� B������1 mol H2O��g����ͬʱ����1 mol H2
C����H���� D��v��(CO) = v��(H2)
��2������ѹǿP����ʼѹǿP0��ʾ��Ӧ��ϵ�������ʵ���n����n����____ mol���ɱ������ݼ��㷴Ӧ��ƽ��ʱ����Ӧ��H2O(g)��ת���ʦ� =_____����ȷ��С�����ڶ�λ����
�����ʼ��仯�����ڹ�ũҵ������������Ҫ��Ӧ�á�
��1����֪25��ʱ��xSO2 (g)��2xCO(g)��2xCO2 (g)��Sx (s) ��H��ax kJ/mol ��
2xCOS(g)��xSO2 (g)��2xCO2 (g)��3Sx (s) ��H��bx kJ/mol�� ��
��ӦCOS(g)����CO(g)��Sx (s)���Ȼ�ѧ����ʽ�� ��
��2��������ʵ���Ũ��Na2S��NaOH�����Һ�еμ�ϡ����������������H2S��HS?��S2?�ķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������μ���������Ĺ�ϵ��ͼ��ʾ�����Եμӹ���H2S������ݳ������Է�����

��B���ߴ��� �����仯(�������ű�ʾ)���μӹ����У���Һ��һ��������
c(Na+)= ��
��M�㣬��Һ����Ҫ�漰�����ӷ���ʽ ��
��14�֣�ÿ��2�֣���
I����1��AD ��2��P/P0 79.82%����0.80��
II����1��xCOS(g) =" xCO(g)+" Sx(s) ��H=0.5(bx��ax)kJ/mol
��2����HS�� c(Na+)=3[c(H2S)+c(HS��)+c(S2��)]���������غ�ó���
��c(Na+)= c(Cl��)+c(OH��)+c(HS��)+2c(S2��)��c(H+)���ɵ���غ�ó���
��M��2S2��+3H+=HS��+H2S
I����1��AD ��2��P/P0 79.82%����0.80��
II����1��xCOS(g) =" xCO(g)+" Sx(s) ��H=0.5(bx��ax)kJ/mol
��2����HS�� c(Na+)=3[c(H2S)+c(HS��)+c(S2��)]���������غ�ó���
��c(Na+)= c(Cl��)+c(OH��)+c(HS��)+2c(S2��)��c(H+)���ɵ���غ�ó���
��M��2S2��+3H+=HS��+H2S
���������I.��1����Ϊ�÷�Ӧ���й�����룬�������������һֱ�仯����������������䣬���Ե����������ܶȲ��ٷ����ı�ʱ��֤���Ѵ�ƽ��״̬����ȷ��B������1 mol H2O��g����ͬʱ����1 mol H2
��������Ӧ������֤����Ӧ��ƽ��״̬������C����Ӧ����ʽ�̶�����Ӧ�Ħ�H�̶������Բ����жϻ�ѧƽ��״̬�Ƿ����D��CO��H2�����ʵ���֮����1:1������v��(CO) = v��(H2)ʱ�������淴Ӧ������ȣ�˵����Ӧ��ƽ��״̬����ȷ����ѡAD��
��2����ʼʱ��������ʵ���Ϊ1mol�����ݺ��º��������£���������ʵ���֮�ȵ��������ѹǿ֮�ȵ�n����P/P0mol���ɱ������ݿ�֪ƽ��ʱ��ѹǿΪ8.20��100Kpa������ʼ��ѹǿΪ4.56��100Kpa����������ˮ���������ʵ���Ϊxmol����ƽ��ʱH2O(g)��CO(g)��H2(g)�����ʵ�����mol���ֱ�Ϊ1-x��x��x������n����P/P0mol����1-x+x+x=8.20/4.56�����x=0.7982�����Է�Ӧ��H2O(g)��ת���ʦ� =79.82%��
II.��1�����ݸ�˹���ɵ�Ŀ�귽��ʽ=����-�٣�/2�����������Ȼ�ѧ����ʽΪxCOS(g) =" xCO(g)+" Sx(s) ��H=0.5(bx��ax)kJ/mol��
��2����������ʵ���Ũ��Na2S��NaOH�����Һ�еμ�ϡ���������������������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ���������Ʒ�Ӧ�������⻯�ƣ���ʱ��Һ��S2?�ĺ�����С��HS?�ĺ������������μ����ᣬ��HS?�������ӽ���������⣬����Һ��H2S�ĺ���������HS?�ĺ�����С������A��B��C�ֱ����S2? ��HS?��H2S�ķ����仯��B���ߴ�����HS?�ķ����仯�����������غ㣬NaԪ�ص�Ũ����SԪ��Ũ�ȵ�3�����ɵõμӹ����У���Һ��һ����������c(Na+)=3[c(H2S)+c(HS��)+c(S2��)]���μӵĹ�������Һ��ʼ�մ���Na+��Cl����HS����S2����OH����H+�����ݵ���غ�ɵ�c(Na+)= c(Cl��)+c(OH��)+c(HS��)+2c(S2��)��c(H+)��
��M���ʾHS����S2��������ȣ�������Һ�з������ܵ����ӷ���ʽΪ2S2��+3H+=HS��+H2S

��ϰ��ϵ�д�
�����Ŀ
 2C(��)�ﵽƽ��ı�־��( )
2C(��)�ﵽƽ��ı�־��( )  2AB(g) ����Ӧ�ﵽƽ��״̬�ı�־��
2AB(g) ����Ӧ�ﵽƽ��״̬�ı�־��  ����
���� a Z��g����
a Z��g����

 3C(s) + 4NH3(g) ��H��0����700�棬CH4��N2�ڲ�ͬ���ʵ���֮��[n(CH4)/n(N2)]ʱCH4��ƽ��ת��������ͼ��ʾ��
3C(s) + 4NH3(g) ��H��0����700�棬CH4��N2�ڲ�ͬ���ʵ���֮��[n(CH4)/n(N2)]ʱCH4��ƽ��ת��������ͼ��ʾ��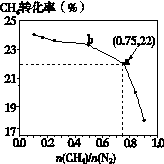
 pC(g)+qQ(g)��m��n��p��qΪ��������ʱ���ﵽƽ��ı�־�ǣ� ��
pC(g)+qQ(g)��m��n��p��qΪ��������ʱ���ﵽƽ��ı�־�ǣ� �� CH3OH(g)
CH3OH(g)