��Ŀ����
9��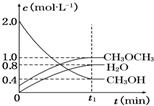 ��������һ����Ҫ�����ȼ�ϣ�����ͨ��CH3OH���Ӽ���ˮ�Ƶã�2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ•mol-1����T1��ʱ�������ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��
��������һ����Ҫ�����ȼ�ϣ�����ͨ��CH3OH���Ӽ���ˮ�Ƶã�2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ•mol-1����T1��ʱ�������ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ������T1��ʱ����Ӧ��ƽ�ⳣ������ֵΪ5��CH3OH��ת����Ϊ80%��
����ͬ�����£����ı���ʼŨ�ȣ�ijʱ�̸����Ũ������Ϊc��CH3OH��=0.4mol•L-1��c��H2O��=0.6mol•L-1��c��CH3OCH3��=1.2mol•L-1����ʱ�����淴Ӧ���ʵĴ�С��v��������v���棩�����������������=������
���� ����ͼ��֪����t1minʱ����ƽ�⣬ƽ��ʱCH3OH��CH3OCH3��H2OŨ�ȷֱ�Ϊ0.4mol/L��1mol/L��0.8mol/L������ƽ�ⳣ������ʽK=$\frac{c��C{H}_{3}OC{H}_{3}����c��{H}_{2}O��}{{c}^{2}��C{H}_{3}OH��}$���㣻ת����=$\frac{Ũ�ȱ仯��}{��ʼŨ��}$��100%��
�ڼ����ʱŨ����Qc����Qc=K������ƽ��״̬����Qc��K����Ӧ������Ӧ���У���Qc��K����Ӧ���淴Ӧ���У������ж������淴Ӧ������Դ�С��
��� �⣺����ͼ��֪����t1minʱ����ƽ�⣬ƽ��ʱCH3OH��CH3OCH3��H2OŨ�ȷֱ�Ϊ0.4mol/L��1mol/L��0.8mol/L������¶���ƽ�ⳣ��K=$\frac{c��C{H}_{3}OC{H}_{3}����c��{H}_{2}O��}{{c}^{2}��C{H}_{3}OH��}$=$\frac{1��0.8}{0��{4}^{2}}$=5��CH3OHת����=$\frac{2mol/L-0.4mol/L}{2mol/L}$��100%=80%��
�ʴ�Ϊ��5��80%��
��Ũ����Qc=$\frac{1.2��0.6}{0��{4}^{2}}$=4.5��K=1����Ӧ������Ӧ���У���v ��������v ���棩��
�ʴ�Ϊ������
���� ���⿼�黯ѧƽ����㡢ƽ�ⳣ������ѧƽ��ͼ��ȣ��Ѷ��еȣ�ע�����ջ�ѧƽ�ⳣ����Ӧ�ã�1���жϷ�Ӧ���еij̶ȣ�2���жϷ�Ӧ����ЧӦ��3���жϷ�Ӧ���еķ���4���������ʵ�ת���ʵȣ�

| A�� | $\frac{Kw}{c��H+��}$=1��10-13mol•L-1����Һ�У�NH4+��Ca2+��Cl-��NO3- | |
| B�� | ʹ��̪���ɫ����Һ�У�Na+��Al3+��SO42-��Cl- | |
| C�� | ����NaClO����Һ�У�H+��NH4+��SO42-��Br- | |
| D�� | ˮ�������c��H+��=1��10-13mol•L-1����Һ�У�NH4+��Na+��AlO2-��CO32- |
������������Ű�Ҫ�������±���
| ��� | ���ϵ����� | ���ʵ���� |
| 1 | ����ʣ�������״̬�²������� | |
| 2 | ����ʣ���������ˮ | |
| 3 | �ǵ���� | |
| 4 | �Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ��������ܵ��� |
| A�� | ͨ����ĵ缫Ϊ��ظ����������ĵ缫��ӦΪ��C3H8-20e-+10O2-�T3CO2+4H2O | |
| B�� | �õ�ص��ܷ�Ӧ�ǣ�C3H8+5O2�T3CO2+4H2O | |
| C�� | ��·��ÿͨ��5mol���ӣ�Լ��5.6L��״���µı��鱻��ȫ���� | |
| D�� | �����ڵ�����У�O2-�ɸ����������� |
 ��
��