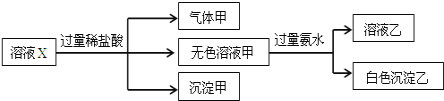
��Ŀ����
����Ŀ���ϳɰ���ҵ���������õ���Fe��������Ҫ��ΪFeO��Fe2O3��
(1)ijFeO��Fe2O3������У������������ʵ���֮��4��5������Fe2����Fe3�����ʵ���֮��Ϊ_____��
(2)��������Fe2����Fe3�������ʵ���֮��Ϊ1��2ʱ�������������ߣ���ʱ�������������������Ϊ______(����2λС��)��
(3)д����C(̿��)��Fe2O3�ڸ����·�Ӧ�Ʊ�������ý�Ļ�ѧ����ʽ(��һ�ֲ��������ˮ)_____��
(4)Ϊ�Ƶ����ֻ�����ߵĴ�����������Ӧ��480 g Fe2O3��ĩ����̿�۵�����Ϊ_____������ʵ��������CO2�����Ϊ_____(�����ʵ�������£�����Ħ�����Ϊ24 L��mol-1)��
���𰸡�1��1 0.72 2Fe2O3��C![]() 4FeO��CO2�� 6 g 12 L
4FeO��CO2�� 6 g 12 L
��������
��1���������������ʵ���֮��4��5����ɵã�
��2���ɴ�����Fe2����Fe3�������ʵ���֮��Ϊ1��2����ɵã�
��3���������Ϣ֪��C�Ὣһ����Fe2O3��ԭ��FeO��ͬʱC������CO2��
��4��������֪������ԭ��Fe2O3��δ����ԭ��Fe2O3�����ʵ���֮��Ϊ1��2��480 g Fe2O3�б���ԭ�����ʵ���Ϊ1mol���ɴ˼���ɵá�
��1����������FeO��Fe2O3�����ʵ����ֱ���a��b���������������ʵ���֮��4��5�ɵã�a+2b������a+3b��=4��5�����a��b=2��1����n��Fe2+����n��Fe3+��=1��1���ʴ�Ϊ��1:1��
��2����Fe2+Ϊ1mol��Fe3+Ϊ2mol���������֪FeԪ�ص�����Ϊ3mol��56g/mol=168g�������������Ϊ1mol��72g/mol+1mol��160g/mol=232g����FeԪ�ص���������Ϊ![]() =0.72���ʴ�Ϊ��0.72��
=0.72���ʴ�Ϊ��0.72��
��3���������Ϣ֪��C�Ὣһ����Fe2O3��ԭ��FeO��ͬʱC������CO2����Ӧ�Ļ�ѧ����ʽΪ2Fe2O3��C![]() 4FeO��CO2�����ʴ�Ϊ��2Fe2O3��C
4FeO��CO2�����ʴ�Ϊ��2Fe2O3��C![]() 4FeO��CO2����
4FeO��CO2����
��4��������֪������ԭ��Fe2O3��δ����ԭ��Fe2O3�����ʵ���֮��Ϊ1��2��480 g Fe2O3�����ʵ���Ϊ![]() =3mol������ԭ��Fe2O3�����ʵ���Ϊ3mol��
=3mol������ԭ��Fe2O3�����ʵ���Ϊ3mol��![]() =1mol���ɷ���ʽ�ɵ�2Fe2O3~C~CO2����C������Ϊ1mol��
=1mol���ɷ���ʽ�ɵ�2Fe2O3~C~CO2����C������Ϊ1mol��![]() ��12g/mol=6g��CO2�����Ϊ1mol��
��12g/mol=6g��CO2�����Ϊ1mol��![]() ��24L/mol=12L���ʴ�Ϊ��6��12��
��24L/mol=12L���ʴ�Ϊ��6��12��

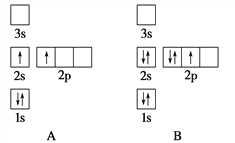
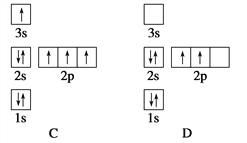
����Ŀ�����ڻ������������⼰ȼú��������������������;�dz��㷺��
![]() ����
����![]() �������
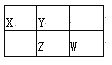
�������![]() ��Ϊ ______ ����������� ______ ������
��Ϊ ______ ����������� ______ ������![]() ѡ�������������������
ѡ�������������������![]() ��
��
![]() Һ����һ��������ϣ���̬��ת��ΪҺ���� ______ ����
Һ����һ��������ϣ���̬��ת��ΪҺ���� ______ ����![]() ѡ��������������ͷ���
ѡ��������������ͷ���![]() Һ����ͨ��ͼ1װ���ͷ��������ù���������ת����ʽΪ ______ ��
Һ����ͨ��ͼ1װ���ͷ��������ù���������ת����ʽΪ ______ ��
![]() ��������Ϊ���������ں��º����ܱ������г���һ������NO��
��������Ϊ���������ں��º����ܱ������г���һ������NO��![]() ����һ�������·�����Ӧ��
����һ�������·�����Ӧ��![]() ��
��
����˵���÷�Ӧ�Ѵﵽƽ��״̬�ı�־�� ______ ![]() ������ѡ��
������ѡ��![]() ��
��
![]() ��Ӧ����
��Ӧ����![]()
![]()
![]()
![]() ������ѹǿ������ʱ��������仯
������ѹǿ������ʱ��������仯
![]() ������
������![]() �����ʵ�������������ʱ��������仯
�����ʵ�������������ʱ��������仯
![]() ������
������![]() ��
��![]() ��
��![]() ��
��![]() ��4��5��6
��4��5��6
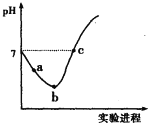
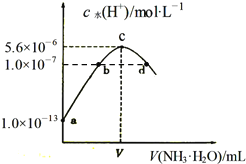
��ij��ʵ���в��������NO��![]() �����ʵ�����ʱ��仯��ͼ2��ʾ��ͼ��
�����ʵ�����ʱ��仯��ͼ2��ʾ��ͼ��![]() ��
��![]() ��
��![]() ��
��![]() ��ȵĵ�Ϊ ______
��ȵĵ�Ϊ ______ ![]() ѡ����ĸ
ѡ����ĸ![]() ��
��

![]() ��֪���Ͽ�1mol���ۼ����յ��������γ�1mol���ۼ��ͷŵ������������
��֪���Ͽ�1mol���ۼ����յ��������γ�1mol���ۼ��ͷŵ������������
���ۼ� |
|
|
|
�����仯 | 436 |
| 946 |
��ϳɰ���Ӧ��![]()
![]() ______
______ ![]()
![]() ��ҵ���ð�ˮ�������Ṥҵβ���е�
��ҵ���ð�ˮ�������Ṥҵβ���е�![]() ���ȿ�������Ⱦ�ֿɻ��
���ȿ�������Ⱦ�ֿɻ��![]() �Ȳ�Ʒ������1000kg ��
�Ȳ�Ʒ������1000kg ��![]() ��������Ϊ
��������Ϊ![]() �İ�ˮ����
�İ�ˮ����![]() ��ȫ��ת��Ϊ
��ȫ��ת��Ϊ![]() �������������ɱ�����ɻ�õ�����Ϊ ______ Ԫ
�������������ɱ�����ɻ�õ�����Ϊ ______ Ԫ![]() ��������ļ۸��
��������ļ۸��![]() ��
��
| ��ˮ | |
�۸� |
|
|
����Ŀ����1����0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ______________������֪��H2O��g��=H2O��l������H2����44.0kJ/mol����11.2L����״������������ȫȼ��������̬ˮʱ�ų���������_____________kJ��
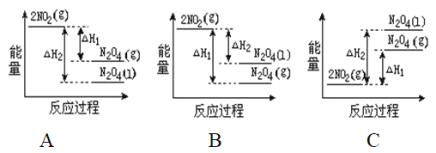
��2����֪��2NO2��g��![]() N2O4��g����H1 2NO2��g��
N2O4��g����H1 2NO2��g��![]() N2O4��l����H2
N2O4��l����H2
���������仯ʾ��ͼ�У���ȷ���ǣ�ѡ����ĸ��_____________��
��3�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C��s��ʯī����O2��g����CO2��g�� ��H1����393.5 kJ��mol��1
2H2��g����O2��g����2H2O��l�� ��H2����571.6 kJ��mol��1
2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H3����2 599 kJ��mol��1
���ݸ�˹���ɣ�����298 Kʱ��C��s��ʯī����H2��g������1 mol C2H2��g����Ӧ���ʱ䣨�г��ļ���ʽ����___________________________��
��4���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�������Ʊ��״����壨�ṹ��ʽΪCH3OH���� ��֪ijЩ��ѧ���ļ����������±���
��ѧ�� | C��C | C��H | H��H | C��O | C��O | H��O |
����/kJ��mol��1 | 348 | 413 | 436 | 358 | 1072 | 463 |
��֪CO�е�C��O֮��Ϊ�������ӣ���ҵ�Ʊ��״����Ȼ�ѧ����ʽΪ_________��