��Ŀ����
3����1��������pH=9��NaOH��Һ��pH=9��CH3COONa��Һ������ˮ���������OH-�����ʵ���Ũ�ȷֱ�ΪA��B����A��B�Ĺ�ϵΪA��B=10-4��1����2���ڴ�����Һ�е����̪����Һ���ɫ�����ڸ���Һ���ٵ��������BaCl2��Һ�����۲쵽�������Dz�����ɫ�������Һ�ɫ��ȥ����ԭ�����ڴ�����Һ��CO32-ˮ�⣺CO32-+H2O?HCO3-+OH-������BaCl2��Ba2++CO32-�TBaCO3������ɫ��������CO32-Ũ�ȼ�С��ˮ��ƽ�����ƣ�OH-Ũ�ȼ�С����̪��ɫ��
��3��������AmBn����Һ��
����Ϊǿ�������Σ���ˮ������ӷ���ʽ��An++nH2O?A��OH��n+nH+��
����Ϊ����ǿ���Σ���ˮ������ӷ���ʽ��Bm-+H2O?HB��m-1��-+OH-��
��4����֪KSP ��Ag2CrO4��=1.12��10-12�����������4��10-3mo1•L-1��AgNO3��4��10-3mo1•L-1K2CrO4��ϣ��ܲ���Ag2CrO4��������ܡ����ܡ�����
���� ��1��������ˮ���룬������������ӵ��δٽ�ˮ���룬��������������Һ��ˮ�������c��OH-�� ����C��H+������������Һ��ˮ�������c��OH-�� ����ˮ���ӻ�������C��H+���ı�ֵ��
��2��̼����ˮ���ʹ��Һ�ʼ��ԣ�̼������Ӻͱ����ӷ�Ӧ����̼�ᱵ����������̼�������ˮ�⣻
��3������������ӽ��ˮ�����������������������������ӣ�
�ڶ�Ԫ��������ӵ�ˮ��ֲ����У���Ҫ�Ե�һ��Ϊ����
��4����������Һ�������ӡ���������ӵ�Ũ�ȣ�Ȼ������ܶȻ�����ʽKSP ��Ag2CrO4��=c2��Ag+��•c��CrO42-�������ݼ������ж��Ƿ����ɳ�����
��� �⣺��1��������������ˮ���룬�����ƴٽ�ˮ���룬��������������Һ��ˮ�������c��OH-������c��H+������������Һ��ˮ�������c��OH-������ˮ���ӻ�������C��H+���ı�ֵ��������������Һ��ˮ�������c��OH-��=10-9 mol/L����������Һ��ˮ�������c��OH-��=$\frac{1{0}^{-14}}{1{0}^{-9}}$mol/L=10-5 mol/L������A��B=10-4��1��
�ʴ�Ϊ��A��B=10-4��1��
��2��������ǿ�������Σ�̼���������ˮ���������������ӣ�������Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ��Һ�ʼ��ԣ�����Һ�м����Ȼ��������Ӻ�̼������ӷ�Ӧ����̼�ᱵ����������̼�������ˮ�⣬��Һ���Լ��������Է�̪��ɫ��
�ʴ�Ϊ��������ɫ�������Һ�ɫ��ȥ���ڴ�����Һ��CO32-ˮ�⣺CO32-+H2O?HCO3-+OH-������BaCl2��Ba2++CO32-�TBaCO3������ɫ��������CO32-Ũ�ȼ�С��ˮ��ƽ�����ƣ�OH-Ũ�ȼ�С����̪��ɫ��
��3������Ϊǿ�������Σ���ˮ������ӷ���ʽΪ��An++nH2O?A��OH��n+nH+��
�ʴ�Ϊ��An++nH2O?A��OH��n+nH+��
��AmBn����ҺΪ����ǿ���ε���Һ����Ԫ�����������ˮ���ҷֲ�ˮ�⣬���һ��ˮ������ӷ���ʽΪ��Bm-+H2O?HB��m-1��-+OH-��
�ʴ�Ϊ��Bm-+H2O?HB��m-1��-+OH-��
��4�����Һ��������Ũ��Ϊ��c��Ag+��=$\frac{1}{2}$��4��10-3mo1•L-1=2��10-3mo1•L-1����������ӵ�Ũ��Ϊ��c��CrO42-��=$\frac{1}{2}$��4��10-3mo1•L-1=2��10-3mo1•L-1��
KSP��Ag2CrO4��=c2��Ag+��•c��CrO42-��=��2��10-3��2��2��10-3=8��10-9��1.12��10-12�������ܹ����ɸ�����������
�ʴ�Ϊ���ܣ�
���� ���⿼��pH�ļ��㡢����ˮ���֪ʶ�㣬��ȷ��Ԫ��������ӷֲ�ˮ�⣬Ϊ�״��㣬ע��������Һ���������ҺpH�ļ��㷽������ȷ�ܶȻ��ı���ʽ�����㷽��������������ѧ���Ļ�ѧ������������Ŀ�Ѷ��еȣ�

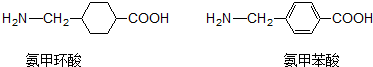
| A�� | �����ᡢ���ױ��ᶼ�ܷ���ȡ�����������Ӿۡ��ӳɵȷ�Ӧ | |
| B�� | �����ᡢ���ױ����ж���5�ֲ�ͬ����ԭ�� | |
| C�� | �������백�ױ��ụΪͬ���칹�� | |
| D�� | �����ᡢ���ױ��ᶼ����NaOH��Һ��Ӧ |
| A�� | O2��O4����ͬ�������� | B�� | H2��H4����ͬλ�� | ||
| C�� | C60��������Ϊ 720 g/mol | D�� | N5+�к���36������ |
| A�� | H2��D2��Ϊͬλ�� | B�� | �⾧�塢��������ͬ�������� | ||
| C�� | NH4OCN��CO��NH2��2��Ϊͬ���칹�� | D�� | C2H4��C3H6һ����ͬϵ�� |

| A�� | �����ʷ���ʽΪC13H14O4 | |
| B�� | ����FeCl3��Һ������ɫ��Ӧ | |
| C�� | ��һ�������¿ɷ����ӳɡ�ȡ������ȥ��Ӧ | |
| D�� | 1 mol�������������2mol NaOH��Ӧ |
| A�� | $\frac{1}{2}$��lgc-lgb�� | B�� | $\frac{1}{2}$��lgb-lgc�� | C�� | $\frac{1}{2}$��lgb-lgc��-a | D�� | $\frac{1}{2}$��lgc-lgb��-a |
| A�� | ��ͬ�ĵ����� | B�� | ��ͬ�������� | ||
| C�� | ������ȫ��ͬ�Ļ�ѧ���� | D�� | ��ͬ�������� |
| A�� | ��ˮ�����Ҵ�������CCl4 | |
| B�� | ��ȼ�շ�������顢��Ȳ��������̼ | |
| C�� | ��̼������Һ�����Ҵ���������������� | |
| D�� | �����Ը��������Һ������Ȳ����ϩ�ͱ��� |
