��Ŀ����
ijͬѧ����ͼװ�ý���ͭ��һ�������֪Ũ�ȵ�Ũ���ᷴӦ��ʵ���о���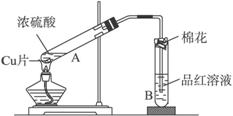
��1��д��A��B���Թ��е�ʵ������
A__________________________________��__________________________________�����ּ��ɣ���B__________________________________��
��2����ַ�Ӧ����ͭ�����ᶼ��ʣ�ࡣ�ڲ�����Ũ�����ǰ���£���ʹʣ��ͭƬ�ܽ⣬���ټ������ʵĻ�ѧʽΪ____________________��
��3���ⶨʣ����������ʵ���ʱ���Ȳ�ͭ��Ũ���ᷴӦ����SO2�������ټ�����������ʵ���������Ϊ�ⶨSO2�����ķ����ж��֣����з����в����е��ǣ� ��
A.��A��������������ͨ��Ԥ�ȳ�����ʢ�м�ʯ�ҵĸ���ܣ�������Ӧ���ٴγ���
B.��A���������建��ͨ��ϡ�����ữ��KMnO4��Һ��������BaCl2��Һ�����ˡ�ϴ�ӡ������������
C.��A���������建��ͨ��������HNO3�ữ��Ba(NO3)2��Һ����ַ�Ӧ���ˡ�ϴ�ӡ������������
D.���ű���NaHSO3��Һ�ķ�����������װ��A����SO2����������������ɱ�״����
��4����Ӧ�����Һ�м�������������ͭ��ʹʣ�������ת��Ϊ����ͭ�����˺���Һ����Ũ������ȴ�Ƶ�����ͭ���壨CuSO4��xH2O�����ü��ȷ��ⶨ�þ�����ᾧˮx��ֵ������һ��ʵ�������Ϊ��
�������� | �����뾧�������� | ���Ⱥ���������������� |
11.7 | G | 11.7 |
�������ݼ��㣬�ж�x��ʵ��ֵ������ֵ��x��5��_________���ƫ��ƫС������ʵ���в�������ԭ�������_________������ĸ��ţ���
A.����ͭ�����к��в��ӷ�����
B.����ʧˮ��¶���ڿ�������ȴ
C.����ʱ�о���ɽ���ȥ
D.����ͭ���壨CuSO4��xH2O���ڳ���ǰ����ı���������ˮ��
��1��ͭƬ�ܽ⣬�����ݲ�����[��Һ�����ɫ����Һ�ɫ��] Ʒ����ɫ
��2��NaNO3 ����Fe2O3�����������𰸾��ɣ�
��3��B
��4��ƫ�� CD
��������1����Cu��Ũ���ᷴӦ�Ľ��ֱ��дA��Ӧ�е�������SO2��Ư����д��B������2���ټ�������ʱ����������ᷴӦ�IJ��Ӧ���ܰ�ͭ�����������������������£��ɽ�ͭ���������������������ᷴӦ�IJ����������ɽ�ͭ��������3��A.ʢ�м�ʯ�ҵĸ���ܷ�Ӧ�����ӵ���������SO2��������B.ͨ��SO2֮ǰ�Ѿ������ᣬ���ɵ����ᱵ��ƫ�ࡣC.SO2ȫ���������������ɵ����ᱵ���ʵ�����ȡ�D.����NaHSO3��Һ�ɽ���SO2���ܽ�ȣ��������ܽ⣬�ռ���������������������ɵ������ȡ�

 ijͬѧ������ͼװ�ý���ʵ�飬֤����ͭ��ϡ���ᷴӦ������NO��ʵ��ʱ������ע�����ڼ���һ������ϡ���ᣬ�ž�ע�����ڵĿ�����Ѹ�ٽ�����ͭ˿����Ƥñ���ϣ�һ��ʱ���ע����������ɫ���������
ijͬѧ������ͼװ�ý���ʵ�飬֤����ͭ��ϡ���ᷴӦ������NO��ʵ��ʱ������ע�����ڼ���һ������ϡ���ᣬ�ž�ע�����ڵĿ�����Ѹ�ٽ�����ͭ˿����Ƥñ���ϣ�һ��ʱ���ע����������ɫ���������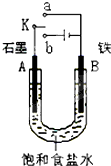 ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣮
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣮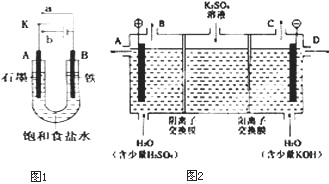 ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺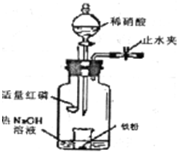 ijͬѧ����ͼװ�ý�������ϡ���ᷴӦ��ʵ��̽����ʵ�鲽��Ϊ��
ijͬѧ����ͼװ�ý�������ϡ���ᷴӦ��ʵ��̽����ʵ�鲽��Ϊ��