��Ŀ����
����ʵ����۴�����ǣ� ��
| A����3��4�����ͷ����ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У���AgNO3��Һ��ϡ�����NaNO2��Һ�������ְ�ɫ������˵��������Ԫ�� |
| B����Ħ����ʱ�þƾ�ϴ�Ӳ�Ʒ |
| C���Ʊ���������茶���ʱ�������������������Ũ����Һʱ��ֻ��С���������Һ������־�ĤΪֹ�����ܽ���Һȫ������ |
| D����ȡ�����е�Ԫ��ʱ��Ϊ��I����ȫ����ΪI2����HNO3������������H2O2��������ˮЧ���� |
D
��

��ϰ��ϵ�д�
�����Ŀ
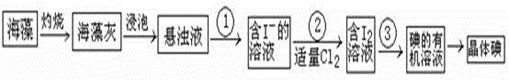
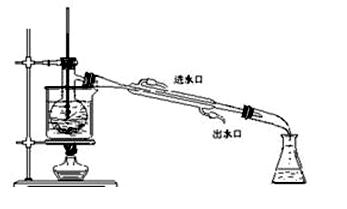

 Na2SO4 + 2HCl�� Ϊԭ������ȡ����HCl���壬�Ծݴ˷������ش��������⣺
Na2SO4 + 2HCl�� Ϊԭ������ȡ����HCl���壬�Ծݴ˷������ش��������⣺
 ��
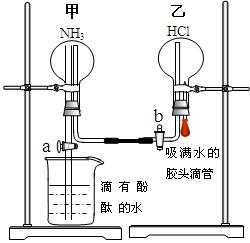
�� ���رջ���b��������a���ֽ����ܹ۲쵽��ʵ�������ǣ� ��
���رջ���b��������a���ֽ����ܹ۲쵽��ʵ�������ǣ� ��