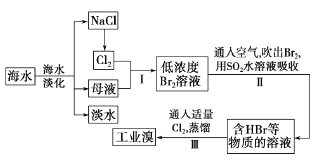
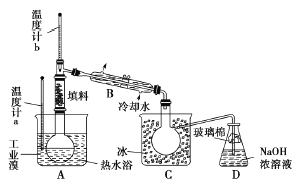
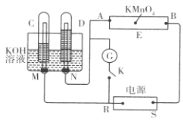
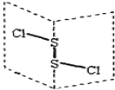
��Ŀ����
����Ŀ��
�� �� | �۵㣨���� | �е㣨���� | �ܶȣ�g/cm3�� |
�� �� | -117.0 | 78.0 | 0.79 |
�� �� | 16.6 | 117.9 | 1.05 |
�������� | -83.6 | 77.5 | 0.90 |
Ũ���ᣨ98%�� | ���� | 338.0 | 1.84 |
ijѧ����ʵ������ȡ������������Ҫ�������£�
����30mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
������ͼ1��ʾ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10����
�����Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ã��ȴ��ֲ㣮
����������������㲢ϴ�ӡ����
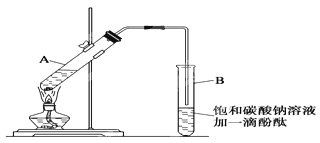
�������ĿҪ��ش��������⣺
��1�����Ƹû����Һ����Ҫ��������Ϊ��______��д����ȡ���������Ļ�ѧ����ʽ��______��
��2������ʵ���б���̼������Һ�������ǣ�______������ĸ����
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С����������ֲ�����
D�������������ɣ���������
��3������������ҪС����ȼ��ȣ�����Ҫ�����ǣ�______��
��4��ָ�����������۲쵽������______������������������һ���ñ���ʳ��ˮ�ͱ����Ȼ�����Һϴ�ӣ���ͨ��ϴ�ӳ�ȥ______�������ƣ����ʣ�Ϊ�˸���������������ѡ�õĸ����Ϊ______������ĸ����
A��P2O5B����ˮNa2SO4C����ʯ�� D��NaOH����
��5��ij��ѧ����С�����������ͼ��ʾ����ȡ����������װ�ã�ͼ�е�����̨�����С�����װ������ȥ��������ͼ��װ����ȣ���ͼװ�õ���Ҫ�ŵ��У�____________��_________����д���������ɣ�

���𰸡���1��Ӧ�ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ����������CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O����2��BC����3�����������Ҵ��Ļӷ������ٸ���Ӧ�ķ�������4���Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz��B����5�����������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����ķ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����������������װ�ã��������ռ���������������
CH3COOCH2CH3+H2O����2��BC����3�����������Ҵ��Ļӷ������ٸ���Ӧ�ķ�������4���Թ�B�е�Һ��ֳ��������㣬�ϲ���ɫ���²�Ϊ��ɫҺ�壬���²�Һ��ĺ�ɫ��dz��B����5�����������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����ķ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����������������װ�ã��������ռ���������������
�������������������1����һ��30mL���Թ���ע��4mL�Ҵ����ٻ�������1mLŨ�����Һ����ȴ���ټ���4mL���ᣬ�ӱ����Թ�ʹ֮��Ͼ��ȣ�ҩƷ���������ܳ���10mL������Ӧ�Ļ�ѧ����ʽ�ǣ�
CH3COOH+ CH3CH2OH![]() CH3COOCH2CH3+H2O����2������������ԣ��ܺͱ���̼������Һ��Ӧ���ѱ����գ����������ڱ���̼������Һ�е��ܽ�Ƚ�С�����ڷ��룬�ʴ�Ϊ��BC����3���Ҵ�������ķе�ϵͣ�Ϊ��ֹ�Ҵ�������ӷ���ӦС����ȣ����������ԭ�ϵ���ʧ���ʴ�Ϊ����Ӧ�����Ҵ�������ķе�ϵͣ����ô����ȣ�������Ӧ���������������ʧԭ�ϣ��¶ȹ������ܷ�����������Ӧ����4��̼����ˮ��ʼ��ԣ��������������ڱ���̼������Һ���ܶȱ�ˮС������ζ����ʱ�����̼���Ʒ�Ӧ��ʹ��Һ��ɫ��dz�����������л���̼���ƺ��Ҵ����ñ���ʳ��ˮ��ȡ̼���ƣ����Ȼ��Ƴ�ȥ�����Ҵ�������ˮ�����Ƴ�ȥ������ˮ����ˮ��������ˮ�γ������ƽᾧˮ�������ѡ��P2O5����ʯ�Һ�NaOH�ȹ����������Է��������������ԣ�P2O5��ˮ�����ᣩ�����������ˮ�⡣�ʴ�Ϊ����dz��ɫ̼������Һ���Ϸ�����ɫҺ����֣��ŵ���ζ����̼������Һ���ɫ��dz��̼���ơ��Ҵ���B����5���Ӹ�װ���봫ͳ�Ʊ�װ�õ�ʹ�õ������IJ�ͬ���з������������¶ȼƣ������ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ݷ�Ӧ�Ŀ������ص㣬���ӷ�Ӧ��������ƽ�����������ƶ���������������IJ���������������װ�ã��������ռ�����ʴ�Ϊ��a���������¶ȼƣ������ڿ��Ʒ���װ���з�ӦҺ���¶ȣ�b�������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����c������������װ�ã��������ռ����
CH3COOCH2CH3+H2O����2������������ԣ��ܺͱ���̼������Һ��Ӧ���ѱ����գ����������ڱ���̼������Һ�е��ܽ�Ƚ�С�����ڷ��룬�ʴ�Ϊ��BC����3���Ҵ�������ķе�ϵͣ�Ϊ��ֹ�Ҵ�������ӷ���ӦС����ȣ����������ԭ�ϵ���ʧ���ʴ�Ϊ����Ӧ�����Ҵ�������ķе�ϵͣ����ô����ȣ�������Ӧ���������������ʧԭ�ϣ��¶ȹ������ܷ�����������Ӧ����4��̼����ˮ��ʼ��ԣ��������������ڱ���̼������Һ���ܶȱ�ˮС������ζ����ʱ�����̼���Ʒ�Ӧ��ʹ��Һ��ɫ��dz�����������л���̼���ƺ��Ҵ����ñ���ʳ��ˮ��ȡ̼���ƣ����Ȼ��Ƴ�ȥ�����Ҵ�������ˮ�����Ƴ�ȥ������ˮ����ˮ��������ˮ�γ������ƽᾧˮ�������ѡ��P2O5����ʯ�Һ�NaOH�ȹ����������Է��������������ԣ�P2O5��ˮ�����ᣩ�����������ˮ�⡣�ʴ�Ϊ����dz��ɫ̼������Һ���Ϸ�����ɫҺ����֣��ŵ���ζ����̼������Һ���ɫ��dz��̼���ơ��Ҵ���B����5���Ӹ�װ���봫ͳ�Ʊ�װ�õ�ʹ�õ������IJ�ͬ���з������������¶ȼƣ������ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ݷ�Ӧ�Ŀ������ص㣬���ӷ�Ӧ��������ƽ�����������ƶ���������������IJ���������������װ�ã��������ռ�����ʴ�Ϊ��a���������¶ȼƣ������ڿ��Ʒ���װ���з�ӦҺ���¶ȣ�b�������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����c������������װ�ã��������ռ����