��Ŀ����
����Ŀ��������ԭ��Ӧ��һ����Ҫ�ķ�Ӧ���ڹ�ũҵ�������ճ������ж��й㷺����;��
��1����ҩ���й��ġ��Ĵ�����֮һ����Զֵ�������セ�����ڻ�ҩ�ڷ�����ըʱ���������·�Ӧ��2KNO3��3C��S��K2S��N2����3CO2�������б�������Ԫ����____________����ԭ������____________��
��2��ʵ����Ϊ�������й������ĺ�������������Ϳ��CuI����ֽ��������ֽ�Ƿ��ɫ����ɫ�����仯����ȥ��ʱ�����жϿ����еĺ��������䷴ӦΪ4CuI��Hg��Cu2HgI4��2Cu��
��������Ӧ����Cu2HgI4�У�CuԪ����________�ۡ�
�����Ϸ�Ӧ�е�������Ϊ________������1 mol CuI���뷴Ӧʱ��ת�Ƶ���________mol��
�۱���������Ӧ����ת�Ƶķ������Ŀ______________________________��
��3����ҵ�ϳ������Ը��������Һ��������CuS��Cu2S�Ŀ���䷴Ӧԭ�����£�
8MnO4����5Cu2S��44H����10Cu2����5SO2����8Mn2����22H2O
6MnO4����5CuS��28H����5Cu2����5SO2����6Mn2����14H2O
����������Ӧԭ����ijѧϰС����400 mL 0.075 mol��L��1�����Ը��������Һ����2 g����CuS��Cu2S�Ļ�����Ӧ�������Һ���Ͼ�SO2��ʣ���KMnO4ǡ����350 mL 0.1 mol��L��1��(NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
����ƽKMnO4��(NH4)2Fe(SO4)2��Ӧ�����ӷ���ʽ��
______MnO4����______Fe2����______H����______Mn2����______Fe3����______H2O��
��KMnO4��Һ��������ﷴӦ��ʣ��KMnO4�����ʵ���Ϊ________mol��
���𰸡�C K2S��N2 ��1 CuI 0.5 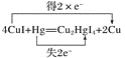 1��5��8��1��5��4 0.007
1��5��8��1��5��4 0.007
��������
��1�����ϼ����ߵ�Ԫ�ر�����������������ԭ�õ���ԭ���
��2���ٸ��ݻ������������۴�����Ϊ0���
������Ԫ�ػ��ϼ۽��͵�������������������Cu��HgԪ�صĻ��ϼ۱仯����ת�Ƶ��ӵ����ʵ�����
�۸���Cu��HgԪ�صĻ��ϼ۱仯��������ת�������
��3���ٸ���Mn��FeԪ�صĻ��ϼ۱仯��ϵ��ӵ�ʧ�غ���
�ڸ��ݷ���ʽ���㡣
��1����Ӧ2KNO3��3C��S��K2S��N2����3CO2����̼Ԫ�ػ��ϼ����ߣ���˱�������Ԫ����C����Ԫ�غ���Ԫ�ػ��ϼ۽��ͣ�����ԭ�����Ի�ԭ������K2S��N2��
��2����Cu2HgI4��Hg��+2�ۣ�I�ǣ�1�ۣ����������۴�����Ϊ0��֪CuԪ����+1�ۡ�
�ڸ��ݷ���ʽ��֪CuI��ͭԪ�ػ��ϼ۴�+1�۽��͵�0�ۣ��õ�1�����ӣ����Է�Ӧ�е�������ΪCuI�����ݷ���ʽ��֪4molCuI�μӷ�Ӧת��2mol���ӣ�����1 mol CuI���뷴Ӧʱ��ת�Ƶ���0.5mol��
�۷�Ӧ��HgԪ�ػ��ϼ۴�0�����ߵ�+2�ۣ�ʧȥ2�����ӣ���������Ӧ����ת�Ƶķ������Ŀ�ɱ�ʾΪ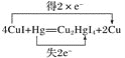 ��
��
��3���ٷ�Ӧ��MnԪ�ػ��ϼ۴�+7�۽��͵�+2�ۣ��õ�5�����ӣ�FeԪ�ػ��ϼ۴�+2�����ߵ�3�ۣ�ʧȥ1�����ӣ����ݵ��ӵ�ʧ�غ㡢ԭ���غ�͵���غ��֪��Ӧ�ķ���ʽΪMnO4����5Fe2����8H����Mn2����5Fe3����4H2O��
�ڷ�Ӧ�������������ӵ����ʵ�����0.35L��0.1mol/L��0.035mol�����ݷ�Ӧ����ʽMnO4����5Fe2����8H����Mn2����5Fe3����4H2O��֪ʣ����������0.035mol��5��0.007mol��

 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�