��Ŀ����
��6�֣���80��ʱ����0.40 mol N2O4�������2 L�Ѿ���յĹ̶��ݻ����ܱ������з�����ӦN2O4��g�� 2NO2��g����H=" +57" kJ��mol��1����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2��g����H=" +57" kJ��mol��1����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
��1������a = �����¶�ʱ�÷�Ӧ��ƽ�ⳣ��K= ��
��2���ı�����ʹ��Ӧ���´ﵽƽ�⣬��ʹc(NO2)/c(N2O4)ֵ��С�Ĵ�ʩ�У�����ţ� ��
A������N2O4����ʼŨ�� B�������¶�
C��ʹ�ø�Ч���� D������������ͨ��ϡ������
 2NO2��g����H=" +57" kJ��mol��1����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2��g����H=" +57" kJ��mol��1����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 | 100 |
| n(N2O4)/mol | 0.40 | a | 0.20 | c | d | e |
| n(NO2)/mol | 0.00 | 0.24 | b | 0.52 | 0.60 | 0.60 |
��2���ı�����ʹ��Ӧ���´ﵽƽ�⣬��ʹc(NO2)/c(N2O4)ֵ��С�Ĵ�ʩ�У�����ţ� ��
A������N2O4����ʼŨ�� B�������¶�
C��ʹ�ø�Ч���� D������������ͨ��ϡ������
��1��a="0.28 " 1.8 mol / L����λ��λ�����֣���2��A
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 2C��g�� ��2���ӷ�Ӧ�ﵽƽ�⣬��ʱ����0.2mol C��
2C��g�� ��2���ӷ�Ӧ�ﵽƽ�⣬��ʱ����0.2mol C��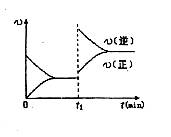
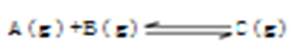 �ﵽƽ���������A��Ũ�Ȼ�����ѹǿʹƽ�������ƶ�������˵������ȷ����
�ﵽƽ���������A��Ũ�Ȼ�����ѹǿʹƽ�������ƶ�������˵������ȷ���� ��KI��Һ�д�������ƽ�⣺
��KI��Һ�д�������ƽ�⣺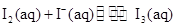 ��ij
��ij
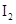 ��KI�����Һ�У�
��KI�����Һ�У� ���¶�T�Ĺ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬������˵������ȷ����
���¶�T�Ĺ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬������˵������ȷ����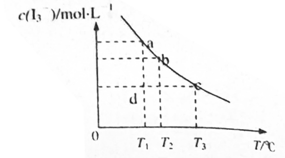

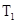 ʱ����Ӧ���е�״̬dʱ��һ����
ʱ����Ӧ���е�״̬dʱ��һ����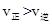
 pZ (g)����H<0���ﻯѧƽ��ı����������б�������ȷ����
pZ (g)����H<0���ﻯѧƽ��ı����������б�������ȷ���� 2NH3��g������H=��QkJ��mo��Q>0����
2NH3��g������H=��QkJ��mo��Q>0����
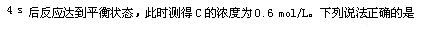
 CH3OH(g)+H2O(g) ��H = ��49.0kJ��mo1��
CH3OH(g)+H2O(g) ��H = ��49.0kJ��mo1��
 mL��pH=11��NaOH��Һ
mL��pH=11��NaOH��Һ mL��Ϸ�Ӧ���ã�������˵������ȷ���� __��
mL��Ϸ�Ӧ���ã�������˵������ȷ���� __��