��Ŀ����
����Ŀ��[2017�¿α���]�̷��Ǻ���һ�����ᾧˮ�������������ڹ�ũҵ�����о�����Ҫ����;��ij��ѧ��ȤС����̷���һЩ���ʽ���̽�����ش��������⣺
��1�����Թ��м��������̷���Ʒ����ˮ�ܽ⣬�μ�KSCN��Һ����Һ��ɫ�����Ա仯�������Թ���ͨ���������Һ��졣�ɴ˿�֪��______________��_______________��
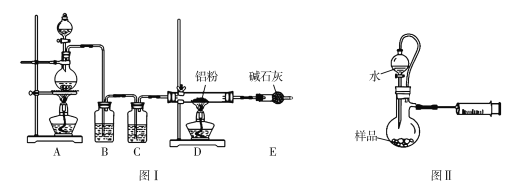
��2��Ϊ�ⶨ�̷��нᾧˮ��������ʯӢ�����ܣ������˿���K1��K2������Ϊװ��A�����أ���Ϊm1 g������Ʒװ��ʯӢ�������У��ٴν�װ��A���أ���Ϊ m2 g������ͼ���Ӻ�װ�ý���ʵ�顣

������B��������____________________��
��������ʵ�����������ȷ����___________________�����ţ����ظ������������裬ֱ��A���أ���Ϊm3 g��
a����ȼ�ƾ��ƣ����� b��Ϩ��ƾ��� c���ر�K1��K2
d����K1��K2������ͨ��N2 e������A f����ȴ������
������ʵ���¼�������̷���ѧʽ�нᾧˮ��Ŀx=________________����ʽ��ʾ������ʵ��ʱ��a��d�����������ʹx__________���ƫ��ƫС������Ӱ�족����
��3��Ϊ̽�����������ķֽ�������2�����Ѻ��ص�װ��A������ͼ��ʾ��װ���У���K1��K2������ͨ��N2�����ȡ�ʵ���Ӧ���в�������Ϊ��ɫ��ĩ��
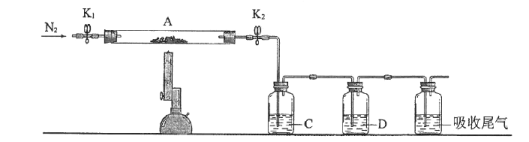
��C��D�е���Һ����Ϊ_________�����ţ���C��D��������ð�������ɹ۲쵽������ֱ�Ϊ_______________��
a��Ʒ�� b��NaOH c��BaCl2 d��Ba(NO3)2 e��ŨH2SO4
��д�������������·ֽⷴӦ�Ļ�ѧ����ʽ_____________________��
���𰸡���1����Ʒ��û��Fe3+ Fe2+�ױ���������ΪFe3+
��2��������� �� dabfce ��![]() ƫС
ƫС
��3����c��a ���ɰ�ɫ��������ɫ
��2FeSO4![]() Fe2O3+SO2��+SO3��
Fe2O3+SO2��+SO3��
����������1����Ʒ����ˮ�μ�KSCN��Һ����Һ��ɫ�����Ա仯��˵����Ʒ����Fe3+�������Թ���ͨ���������Һ��죬��˵���������Ӳ����������������ױ���������Ϊ����������������KSCN��Һ�Ժ�ɫ��
��2�����������������֪B�Ǹ���ܡ�
������װ���к��п����������������������������Լ���ǰ��Ҫ�ž�װ���п��������õ����ų�������Ϊ��ʹ���ɵ�ˮ������ȫ�ų���Ӧ����Ϩ��ƾ��ƣ�����ȴ��Ȼ��ر�K1��K2��������������ȷ��������dabfce��
����Ʒ��������(m2��m1)g�����Ⱥ�ʣ���������������������Ϊ(m3��m1)g������ˮ������Ϊ(m2��m3)g��
FeSO4��xH2O![]() FeSO4 + xH2O
FeSO4 + xH2O
152 18x
��m3��m1��g ��m2��m3��g
��![]() ����ã�
����ã�![]() ��
��
��ʵ��ʱ��a��d����������ڼ��ȹ����в���������������������Ϊ������������m3���ӣ�ʹ������ɵ�ˮƫС�����ɵ���������ƫ�����xƫС��
��3�������յõ���ɫ��ĩ��˵�������������ɣ����ֽ���̷�����������ԭ��Ӧ�����ݻ��ϼ۱仯��֪һ����SO2���ɣ���˵�����������ֽ�������������SO2����������������������ˮ�������ᣬ����ͱ����ӽ�����ɰ�ɫ�������ᱵ���������ᱵ��������Һ���������ԣ�������SO2������Ӧ�����Ȼ���������SO2��Ʒ����Һ������C��D����Һ����Ϊ�Ȼ�����Һ��Ʒ����Һ��ʵ��������C����Һ����ǣ�������ɫ������D��Ʒ����Һ��ɫ��
���������Ϸ�����֪�����������·ֽ�������������SO2��SO3�����ݵ����غ��ԭ���غ�ô˷�Ӧ�ķ���ʽΪ2FeSO4![]() Fe2O3+SO2��+SO3����
Fe2O3+SO2��+SO3����

����Ŀ��Ϊ���ᴿ�������ʣ�������Ϊ���ʣ��������Լ��ͷ��뷽������ȷ����
ѡ�� | ���ᴿ������ | �����Լ� | ���뷽�� |
A | �Ҵ�(�Ҵ���) | Na | ���� |
B | �屽(Br2) | �� | ��ȡ |
C | ������(NaCl) | ˮ | �ؽᾧ |
D | ��(����) | NaOH��Һ | ���� |
A. A B. B C. C D. D