��Ŀ����
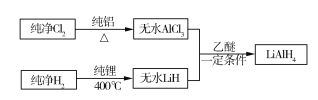
����Ŀ���⻯��ﮣ�LiAlH4�����л��ϳ��зdz���Ҫ�Ļ�ԭ���������⻯����ǰ�ɫ��״���壬���������࣬�������ѣ���120��C���¸������������ȶ�������ˮ����ը�Էֽ⡣ij������ͬѧ�������ϣ�����������̺ϳ��⻯��ﮣ�
��ش��������⣺
��1���⻯�����ˮ�������ҷ�Ӧ��д���⻯�����ˮ��Ӧ�Ļ�ѧ����ʽ��____________________��
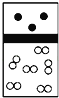
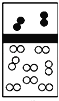
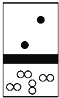
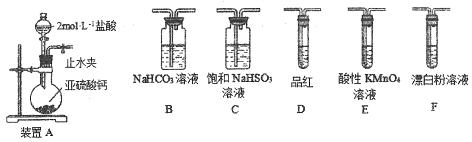
��2���о�С���ͬѧ����ͼIװ����ʵ��������ȡ��ˮ�Ȼ�����
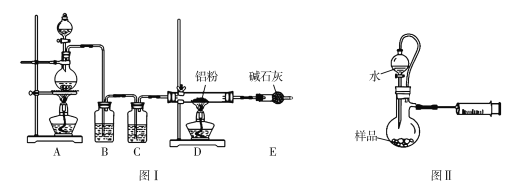
����ѡ��Aװ����ȡ����������ѡ�õ�ҩƷ��_________�����ţ�����Ӧ�����ӷ���ʽΪ_______________________________��
A��Ũ����+KMnO4 B��Ũ����+MnO2
C��Ũ����+KClO3 D��Ca(ClO)2+Ũ����
����Ӧ��ʼʱ��Ӧ���ȵ�ȼ____________�����A����D�����ƾ��ƣ�ֱ���۲쵽___________________________���ٵ�ȼ��һ���ƾ��ơ�
��ͼ��װ��E��������________________________________��
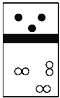
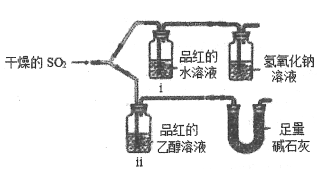
��3���о�С����ͬѧȷ����a g��Ʒ������ƿ�У�����ͼIIװ�ò�����Ʒ��ˮ��Ӧ���ɵ���������������⻯��﮵Ĵ��ȣ��Է����䷽���Ƿ����____________�������������������������������˵������_______________________________________�������������ղ������𣩡�
���𰸡���1��LiAlH4+2H2O![]() LiAlO2+4H2��
LiAlO2+4H2��
��2����B MnO2+4H++2Cl![]() Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O
��A װ��D��Ӳ�ʲ������г�������ɫ����
�����ն������������������е�ˮ��������D��
��3�������� �⻯�����ˮ�ᱬը�Էֽ⣬����Ʒ�л��е�LiH��ˮ��ӦҲ�����H2
����������1���⻯�����ˮ��Ӧ�ų������ȣ�������������ȼ���壬������Ե��±�ը���⻯��﮺�ˮ��Ӧ�ķ���ʽΪ��LiAlH4+2H2O![]() LiAlO2+4H2����
LiAlO2+4H2����
��2����������������Ũ����Ͷ������̷�Ӧ��ȡ��������ѡ��B����Ӧ�����ӷ���ʽΪ��MnO2+4H++2Cl![]() Mn2++Cl2��+2H2O����Ӧ���ȵ�ȼA���ƾ��ƣ���ȡ������ֱ��Dװ�õ�Ӳ�ʲ������г�������ɫ���壬�ٵ�ȼD���ƾ�����ȡ�Ȼ�������װ�м�ʯ�ҵĸ��������������ã�һ������δ��Ӧ�����������Ƿ�ֹ�����е�ˮ��������D�����Ȼ�����Ӧ��
Mn2++Cl2��+2H2O����Ӧ���ȵ�ȼA���ƾ��ƣ���ȡ������ֱ��Dװ�õ�Ӳ�ʲ������г�������ɫ���壬�ٵ�ȼD���ƾ�����ȡ�Ȼ�������װ�м�ʯ�ҵĸ��������������ã�һ������δ��Ӧ�����������Ƿ�ֹ�����е�ˮ��������D�����Ȼ�����Ӧ��
��3�����������⻯�����ˮ�ᱬը�Էֽ⣬��ʵ�鷽������ȫ������Ʒ�к���LiH��LiH��H2O������Ӧ��LiH+H2O![]() LiOH+H2����Ҳ����������������������Ʒ���ȡ�
LiOH+H2����Ҳ����������������������Ʒ���ȡ�

����Ŀ��ij��ѧ��ȤС���ڿ����У���ijһ����Һ�ɷ�(��֪����������ԭ������)�����˼�⣬�������μ�������±���ʾ��
������ | ��Һ�м������������ |
��һ�� | KCl��K2SO4��Na2CO3��NaCl |
�ڶ��� | KCl��AlCl3��Na2SO4��K2CO3 |
������ | Na2SO4��KCl��K2CO3��NaCl |
����˵����������(����)
A. ���μ��������ȷ
B. ����Һ�е����������ж�
C. Ϊ�˼���SO![]() ��Ӧ�ȼӹ���ϡ������ٵμ�Ba(NO3)2���۲��Ƿ��г�������
��Ӧ�ȼӹ���ϡ������ٵμ�Ba(NO3)2���۲��Ƿ��г�������
D. Ϊ��ȷ���Ƿ����CO![]() �����������еμ�CaCl2��Һ���۲��Ƿ��г�������
�����������еμ�CaCl2��Һ���۲��Ƿ��г�������