��Ŀ����
 ��1��15.6g Na2X��Na+0.4moL����Na2X��Ħ������Ϊ
��1��15.6g Na2X��Na+0.4moL����Na2X��Ħ������Ϊ��2�������£���һ�������������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬�õ�200mL��c��OH-��=0.1mol?L-1����Һ��Ȼ����μ���1mol?L-1�����ᣬ������ɳ���������m��������������V��ϵ����ͼ��ʾ��
��ͼ��V1Ϊ
��ԭ�Ͻ����Ƶ�����Ϊ
��ԭ�Ͻ���ˮ��Ӧ���ɵ����干��
���㣺�йػ���ﷴӦ�ļ���,���ʵ�������ؼ���,��ѧ����ʽ���йؼ���
ר�⣺������
��������1��15.6g Na2X��Na+0.4moL����Na2X�����ʵ���Ϊ0.2mol������M=
����û������Ħ��������Ħ����������ֵ�ϵ�������Է������������ԭ��������
��2�������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬����2Na+2H2O�T2NaOH+H2����2Al+2H2O+2NaOH�T2NaAlO2+3H2����������ʱ����NaOH+HCl�TNaCl+H2O��NaAlO2+HCl+H2O�TNaCl+Al��OH��3����Al��OH��3��+3HCl�TAlCl3+3H2O���ٽ��ͼ���м���40mL�������ɵij�����������㣮
| m |
| n |
��2�������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬����2Na+2H2O�T2NaOH+H2����2Al+2H2O+2NaOH�T2NaAlO2+3H2����������ʱ����NaOH+HCl�TNaCl+H2O��NaAlO2+HCl+H2O�TNaCl+Al��OH��3����Al��OH��3��+3HCl�TAlCl3+3H2O���ٽ��ͼ���м���40mL�������ɵij�����������㣮
���
�⣺��1��15.6g Na2X��Na+0.4moL����Na2X�����ʵ���Ϊ0.2mol���û������Ħ������M=
=
=78g/mol��X�����ԭ������=78-23��2=32��
�ʴ�Ϊ��78g/mol��32��
��2������ͼ���֪����Ͻ��ܽ�����Һ�м����ᣬ�ȷ���NaOH+HCl�TNaCl+H2O������NaAlO2+HCl+H2O�TNaCl+Al��OH��3���������Al��OH��3��+3HCl�TAlCl3+3H2O���Ͻ��ܽ��ʣ����������Ƶ����ʵ���Ϊ0.02L��1mol/L=0.02mol��
��NaOH+HCl�TNaCl+H2O��
0.02mol 0.02mol
��V1Ϊ
=0.02L=20mL��
�ʴ�Ϊ��20��
�ڵ��������ʱ����Һ�е�����ΪNaCl��������ԭ�ӡ���ԭ���غ��n��Na��=n��NaCl��=n��HCl��=1mol/L��
0.04L=0.04mol�������Ƶ�����=0.04mol��23g/mol=0.92g��
�ʴ�Ϊ��0.92��
�۸�����ԭ���غ������n��NaOH��=0.04mol��������Һ�д��ڵ�n��NaOH��=0.1mol/L��0.2L=0.02mol��������ԭ���غ��n��NaAlO2��=0.02mol������Alԭ���غ��n��Al��=0.02mol���Ͻ��ˮ��Ӧʱת�Ƶ�����ȣ����������������=
=0.05mol��
�ʴ�Ϊ��0.05��
| m |
| n |
| 15.6g |
| 0.2mol |
�ʴ�Ϊ��78g/mol��32��
��2������ͼ���֪����Ͻ��ܽ�����Һ�м����ᣬ�ȷ���NaOH+HCl�TNaCl+H2O������NaAlO2+HCl+H2O�TNaCl+Al��OH��3���������Al��OH��3��+3HCl�TAlCl3+3H2O���Ͻ��ܽ��ʣ����������Ƶ����ʵ���Ϊ0.02L��1mol/L=0.02mol��
��NaOH+HCl�TNaCl+H2O��
0.02mol 0.02mol
��V1Ϊ
| 0.02mol |
| 1mol/L |
�ʴ�Ϊ��20��
�ڵ��������ʱ����Һ�е�����ΪNaCl��������ԭ�ӡ���ԭ���غ��n��Na��=n��NaCl��=n��HCl��=1mol/L��
0.04L=0.04mol�������Ƶ�����=0.04mol��23g/mol=0.92g��
�ʴ�Ϊ��0.92��
�۸�����ԭ���غ������n��NaOH��=0.04mol��������Һ�д��ڵ�n��NaOH��=0.1mol/L��0.2L=0.02mol��������ԭ���غ��n��NaAlO2��=0.02mol������Alԭ���غ��n��Al��=0.02mol���Ͻ��ˮ��Ӧʱת�Ƶ�����ȣ����������������=
| 0.04mol��1+0.02mol��3 |
| 2 |
�ʴ�Ϊ��0.05��
���������⿼���˻����ļ��㣬��ȷͼ���и����յ���Һ�е������ǽⱾ��ؼ����ٽ��ԭ���غ㡢ת�Ƶ����غ���н����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���й���ŨHNO3��ŨH2SO4��������ȷ���ǣ�������
| A�������¶����������������� |
| B�������¶�����ͭ�Ͽ췴Ӧ |
| C��¶���ڿ����У���Һ���������� |
| D��¶���ڿ����У���ҺŨ�Ⱦ����� |
���е��뷽��ʽ����ȷ���ǣ�������
| A��Na2HPO4����ˮ��Na2HPO4=2Na++H++PO43- |
| B��NaHSO4�ۻ���NaHSO4=Na++H++SO42- |
| C��HF��������ˮ�У�HF=H++F- |
| D����NH4��2SO4����ˮ����NH4��2SO4=2NH4++SO42- |
����˵����ȷ���ǣ�������
| A��ʵ�����еĽ����ͨ��������ʢ��ú�͵��Լ�ƿ�У��Ա����������ˮ |
| B��150mL 1mol?L-1NaCl��Һ��75mL 1mol?L-1 NaHCO3��Һ��Na+���ʵ������ |
| C����FeCl3��Һ�еμ�KSCN��Һ�����Ѫ��ɫ���� |
| D���ýྻ�IJ�˿պȡ��Ʒ�ڻ��������տ�����ɫ���棬˵����Ʒ��һ������Ԫ�� |
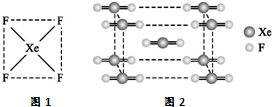 ���Դ�Ӣ����ѧ�Ұ����У�N��Bartlett���״κϳ��˵�һ��ϡ������Ļ�����XePtF6��������������̷�����믵�һϵ�л������XeF2��XeF4�ȣ�������Ϊ����ϡ�����廯ѧ��������ʷ�Թ��ף�
���Դ�Ӣ����ѧ�Ұ����У�N��Bartlett���״κϳ��˵�һ��ϡ������Ļ�����XePtF6��������������̷�����믵�һϵ�л������XeF2��XeF4�ȣ�������Ϊ����ϡ�����廯ѧ��������ʷ�Թ��ף�