��Ŀ����
����Ŀ��A��B��C��D��E��Ϊ������Ԫ�أ�ԭ������������������ݱ�����Ϣ�ش��������⣺
Ԫ�� | Ԫ�����ʻ�ṹ |
A | ���������������ڲ��������2�� |
B | BԪ�صĵ����ڿ����к������ |
C | CԪ���ڵؿ��к������ |
D | D��ͬ������ԭ�Ӱ뾶��С��Ԫ�� |
E | EԪ����ͬ�����н�������ǿ |
F | FԪ�ص����������������������� |
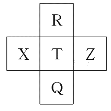
G | GԪ��ԭ��M���ϵ�������L����2�����ӡ� |
(1)G��Ԫ�����ڱ��е�λ��________��
(2)B�����̬�⻯��Ľṹʽ____������____������(��������������������)��E������������ˮ�������ʽ____��������ѧ������________��
(3)C��D��E��F��G�����Ӱ뾶�ɴ�С˳��Ϊ��____(�����ӷ���)��
(4)�õ���ʽ��ʾG���⻯����γɹ���______��
(5)AԪ�ص�ij��������EԪ�ص�ij���������ﷴӦ���ɵ��ʵĻ�ѧ����ʽ��_______��
(6)E��F��Ԫ������������Ӧˮ�������Ӧ�����ӷ���ʽ��____��
���𰸡���3���ڵڢ�A�� ![]() ����
���� ![]() ���Ӽ����ۼ� r(S2-)>r(O2-)>r(F-)>r(Na+)>r(Al3+)
���Ӽ����ۼ� r(S2-)>r(O2-)>r(F-)>r(Na+)>r(Al3+) ![]() 2Na2O2+2CO2=2Na2CO3+O2 Al(OH)3 + OH- = AlO2- + 2H2O
2Na2O2+2CO2=2Na2CO3+O2 Al(OH)3 + OH- = AlO2- + 2H2O
��������
A��B��C��D��E��Ϊ������Ԫ�أ�ԭ��������������A���������������ڲ��������2������AΪC��BԪ�صĵ����ڿ����к�����࣬��BΪN��CԪ���ڵؿ��к�����࣬��CΪO��D��ͬ������ԭ�Ӱ뾶��С��Ԫ�أ���DΪF��EԪ����ͬ�����н�������ǿ����EΪNa��FԪ�ص���������������������������FΪAl��GԪ��ԭ��M���ϵ�������L����2�����ӣ���GΪS��
(1)GΪS����Ԫ�����ڱ��е�λ�õ�3���ڵ���A�壻�ʴ�Ϊ����3���ڵ���A�塣
(2)BΪN�������̬�⻯��ΪNH3����ṹʽ![]() �����ڹ��ۻ����EΪNa��������������ˮ����ΪNaOH�������ʽ
�����ڹ��ۻ����EΪNa��������������ˮ����ΪNaOH�������ʽ![]() ��������ѧ���������Ӽ����ۼ����ʴ�Ϊ��
��������ѧ���������Ӽ����ۼ����ʴ�Ϊ��![]() �����ۣ�
�����ۣ�![]() �����Ӽ����ۼ���
�����Ӽ����ۼ���
(3)���ݲ�ྶ��ͬ���Ӳ�ṹ�˶ྶСԭ����C��D��E��F��G�����Ӱ뾶�ɴ�С˳��Ϊ��r(S2)��r(O2)��r(F��)��r(Na+)��r(Al3+)���ʴ�Ϊ��r(S2)��r(O2)��r(F��)��r(Na+)��r(Al3+)��
(4)�õ���ʽ��ʾG���⻯�TH2S���γɹ��̣����дԭ�ӵĵ���ʽ���ұ�д������ĵ���ʽ��������γɹ���Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)AԪ�ص�ij��������EԪ�ص�ij���������ﷴӦ���ɵ����Ƕ�����̼��������Ʒ�Ӧ����̼���ƺ��������仯ѧ����ʽ��2Na2O2+2CO2=2Na2CO3+O2���ʴ�Ϊ��2Na2O2+2CO2= 2Na2CO3+O2��
(6)E��F��Ԫ������������Ӧˮ�����������ƺ�����������Ӧ����ƫ�����ƺ�ˮ�������Ӧ�����ӷ���ʽ��Al(OH)3 + OH�� = AlO2�� + 2H2O���ʴ�Ϊ��Al(OH)3 + OH�� = AlO2�� + 2H2O��