��Ŀ����
�±������ۺ�������
| ѡ�� | ��ѧ��Ӧ�������ӷ���ʽ | ���� |
| A | ����ϡ���ᷴӦ�� 2Fe+6H+��2Fe3++3H2�� | ��ȷ |
| B | ����ʯ���ڴ���ķ�Ӧ�� CaCO3+ 2H+��Ca2++CO2��+H2O | ������ӦдΪ������ʽCH3COOH��CaCO3Ӧд��������ʽ |
| C | ��Ba(OH)2��Һ�еμ�����NaHSO4��Һ��Ba2+ + OH- + H+ + SO42- = BaSO4��+ H2O | ���� |
| D | NH4HCO3��Һ�����KOHŨ��Һ���ȣ�NH4++ OH�� NH3��+ H2O NH3��+ H2O | ����HCO3-Ҳ������OH����Ӧ |
D
���������Aѡ����Ӧ����Fe2+������Bѡ�������д���ӦдΪ���ӣ�CaCO3Ӧд�ɻ�ѧʽ������Cѡ�����۴�����ʽ��Ba2+��OH-��ѧ������֮��Ϊ1:1������Dѡ����ȷ��

��ϰ��ϵ�д�
 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
�����Ŀ
 2OH- +Cl2��+ H2��
2OH- +Cl2��+ H2�� == CO2����H2O
== CO2����H2O CH3COO��+ NH4+ +3NH3 + 2Ag��+ H2O
CH3COO��+ NH4+ +3NH3 + 2Ag��+ H2O Mn2����2Cl����Cl2����2H2O
Mn2����2Cl����Cl2����2H2O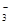 +Ca2��+OH��= CaCO3��+H2O
+Ca2��+OH��= CaCO3��+H2O CH3COOC2H5
CH3COOC2H5