��Ŀ����
�������ƾ���[CaO2��8H2O]���ȶ����ʰ�ɫ������ˮ��������������Һ���㷺Ӧ���ڻ���ɱ��������������
��������ƾ�����Ʊ�
��ҵ������CaO2��8H2O����Ҫ�������£�

��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2������ʱ���ñ�ˮ�����¶���10�����º�ͨ�������NH3�������ԭ��ֱ���
�� ���� ��
��������ƾ��庬���IJⶨ
ȷ��ȡ0.3000g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.0200mol��L��1 KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4����5 H2O2��6H+��2Mn2+��5O2����8H2O
��3���ζ��յ�۲쵽������Ϊ ��
��4�����ݱ�1���ݼ����Ʒ��CaO2��8H2O������������д��������̣���
��1. KMnO4����Һ�ζ�����
��������ƾ�����Ʊ�
��ҵ������CaO2��8H2O����Ҫ�������£�

��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2������ʱ���ñ�ˮ�����¶���10�����º�ͨ�������NH3�������ԭ��ֱ���
�� ���� ��
��������ƾ��庬���IJⶨ
ȷ��ȡ0.3000g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.0200mol��L��1 KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4����5 H2O2��6H+��2Mn2+��5O2����8H2O
��3���ζ��յ�۲쵽������Ϊ ��
��4�����ݱ�1���ݼ����Ʒ��CaO2��8H2O������������д��������̣���
| �ζ����� | ��Ʒ������/g | KMnO4��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.3000 | 1.02 | 24.04 |
| 2 | 0.3000 | 2.00 | 25.03 |
| 3 | 0.3000 | 0.20 | 23.24 |
��1. KMnO4����Һ�ζ�����
��1��CaCl2+H2O2+2NH3+8H2O=CaO2��8H2O��+2NH4Cl����2�����¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ����ֹ��������ķֽ⣩����ͨ�������NH3ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣨����߲�Ʒ�IJ��ʣ�����3�����������һ��KMnO4����Һ������ɫ��dz��ɫ���Ұ�����ڲ���ɫ����4��82.91% 5 CaO2��8H2O �� 5 H2O �� 2 KMnO4
n(CaO2��8H2O)=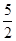 n(KMnO4)=
n(KMnO4)= 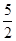 ��0.0200mol/L��23.03mL��10-3L/mL=1.1151��10-3mol������CaO2��8H2O������������
��0.0200mol/L��23.03mL��10-3L/mL=1.1151��10-3mol������CaO2��8H2O������������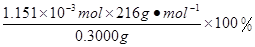 =82.91%
=82.91%
n(CaO2��8H2O)=
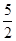 n(KMnO4)=
n(KMnO4)= 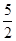 ��0.0200mol/L��23.03mL��10-3L/mL=1.1151��10-3mol������CaO2��8H2O������������
��0.0200mol/L��23.03mL��10-3L/mL=1.1151��10-3mol������CaO2��8H2O������������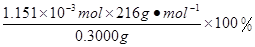 =82.91%
=82.91%�����������1�����������֪��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��CaCl2+H2O2+2NH3+ 8H2O= CaO2��8H2O��+2NH4Cl����2������ʱ���ñ�ˮ�����¶���10�����º�ͨ�������NH3����Ϊ���¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ���ͨ�������NH3ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣨����߲�Ʒ�IJ��ʣ�����3��CaO2+2H2O=Ca(OH)2+H2O2; 2MnO4����5 H2O2��6H+��2Mn2+��5O2����8H2O.���ﵽ�ζ��յ�ʱ��۲쵽��Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ����4���ɷ���ʽ�ù�ϵʽΪ��5CaO2��8H2O ��2KMnO4�����ĵ�KMnO4��Һ�����Ϊ{(24.04��1.02)+( 25.03��2.00)+( 23.24��0.20)}ml��3=23.03ml.n(CaO2��8H2O)=
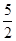 n(KMnO4)=
n(KMnO4)= 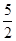 ��0.0200mol/L��23.03mL ��10-3L/mL ="1.1151" ��10-3mol ������CaO2��8H2O������������
��0.0200mol/L��23.03mL ��10-3L/mL ="1.1151" ��10-3mol ������CaO2��8H2O����������Ϊ��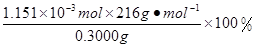 =82.91%��2��8H2O���Ʊ�ԭ��������ע�����⡢�ζ��յ���жϡ����ʺ����IJⶨ��֪ʶ��
=82.91%��2��8H2O���Ʊ�ԭ��������ע�����⡢�ζ��յ���жϡ����ʺ����IJⶨ��֪ʶ��
��ϰ��ϵ�д�
�����Ŀ
 ��Fe(NO3)3��NO����2H2O
��Fe(NO3)3��NO����2H2O
 Cr2O72��+ H2O
Cr2O72��+ H2O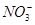 ��Fe3����
��Fe3����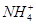 ��H����H2O�������ӣ�����ͬһ������ԭ��Ӧ�еķ�Ӧ��������������������ȷ����
��H����H2O�������ӣ�����ͬһ������ԭ��Ӧ�еķ�Ӧ��������������������ȷ����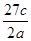
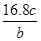


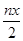 mol
mol