��Ŀ����
����Ŀ��������ᾭ�õķ�չ����������ˮƽ����ߺͶԻ���Ҫ��ļ�ǿ����Դ�㷺�ĸ߰�����ˮ(��Ҫ����NH4+)����Խ��Խ�ܵ����ӡ����ڸ߰�����ˮ�Ĵ����ж��ַ�����
��1�����ѷ�:
![]()
ʹ�ô��ѷ�ʱ��Ҫ�ڢ��м���д�����������ӷ�Ӧ����ʽ______________________��
��2��MAP��������
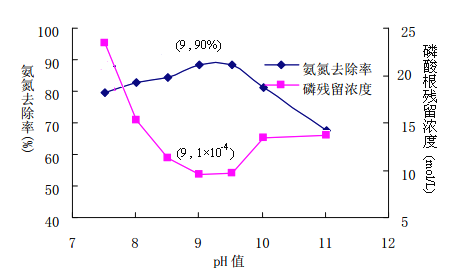
��ʹ�û�ѧ�����������߰�����ˮʱ����߰�����ˮ��Ͷ�뺬��Mg2+�����ʺ�H3PO4��������ҺpH����NH4+��Ӧ����MgNH4PO4(MAP)������Ϊ��Ч������ҺPH����������ˮ����Ч���������ѡ������������_____��
A MgO B MgSO4 C MgCl2
��������ҺPH�ĺ�����ΧΪ____________________
�����ܽ�ƽ��ǶȽ���PH������Ͳ����γɳ���MAP��ԭ����֪PO43-�����Խ�ǿ��������HPO42-��ʽ���ڣ�_______________
��3�������ѵ���ͳ���գ�
�����������������£������ں��������Ҫ�Ǻ�����������������NO3-��д����Ӧ�����ӷ���ʽ_________________________��
�������������������£����������������������������ʹNO3-��״���������N2���ﵽ����ˮ��Ŀ�ġ�д�����ӷ���ʽ____________________________��
���𰸡� NH4++OH-=NH3��H2O A 9-9.5��9-10Ҳ���� ��ϵ�д���ƽ��MgNH4PO4(s)![]() Mg2++NH4++PO43- ����PH����ʱPO43-+H+=HPO42-,c(PO43-)���ͣ�ƽ�����ƣ����������ɳ�������PH����ʱ Mg2++2OH-=Mg(OH)2��,c(Mg2+)���ͣ�ƽ�����ƣ����������ɳ���
Mg2++NH4++PO43- ����PH����ʱPO43-+H+=HPO42-,c(PO43-)���ͣ�ƽ�����ƣ����������ɳ�������PH����ʱ Mg2++2OH-=Mg(OH)2��,c(Mg2+)���ͣ�ƽ�����ƣ����������ɳ��� ![]()
![]()
��������(1) �߰�����ˮ(��Ҫ����NH4+)�Ӽ������Ӧ�����ӷ�Ӧ����ʽΪNH4++OH-=NH3��H2O��
��2����ʹ�û�ѧ�����������߰�����ˮʱ����߰�����ˮ��Ͷ�뺬��Mg2+�����ʺ�H3PO4��������ҺpH����NH4+��Ӧ����MgNH4PO4(MAP)������Ϊ��Ч������ҺPH����������ˮ����Ч���������ѡ-��MgO������ˮ�Լ��ԣ��ֲ����������ʣ���MgSO4 ��MgCl2������SO42- Cl-�������ӣ��ʲ�ѡ�����Դ�ΪA��
����ͼ���������PHΪ9-9.5ʱ������ȥ������ߣ��ײ���Ũ����ͣ����Կ�����ҺPH Ϊ9-9.5Ϊ������Χ��
����ϵ�д���ƽ��MgNH4PO4(s)![]() Mg2++NH4++PO43- ����PH����ʱPO43-+H+=HPO42-,c(PO43-)���ͣ�ƽ�����ƣ����������ɳ�������PH����ʱ Mg2++2OH-=Mg(OH)2��,c(Mg2+)���ͣ�ƽ�����ƣ����������ɳ��� ��
Mg2++NH4++PO43- ����PH����ʱPO43-+H+=HPO42-,c(PO43-)���ͣ�ƽ�����ƣ����������ɳ�������PH����ʱ Mg2++2OH-=Mg(OH)2��,c(Mg2+)���ͣ�ƽ�����ƣ����������ɳ��� ��
��3�������������ں��������Ҫ�Ǻ������������ý�NH4+����NO3-����Ӧ�����ӷ���ʽΪ![]()
�������������������£����������������������������ʹNO3-��״���������N2���ﵽ����ˮ��Ŀ�ġ��䷴Ӧ�����ӷ���ʽ![]() ��
��

����Ŀ����50 mL 1.0 mol/L�����50 mL 1.1 mol/L ����������Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ__________(����ƫ��ƫС����Ӱ����)�������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к���____________(����ƫ��ƫС����Ӱ����)�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬���˲������裬���õ��к��Ȼ�____________(����ƫ��������ƫС������������)��
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�____________(����ƫ��������ƫС������������)����ԭ����_______________________________________________��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50 mL������¼��ԭʼ����(���±�)��
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�(t2)/�� | �²�(t2��t1)/�� | ||
���� | NaOH��Һ | ƽ��ֵ | |||
1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3���кͺ���Һ�ı�����c��4.18��10��3kJ/(g����)����÷�Ӧ���к���Ϊ��H��_______�����ݼ�������д�����кͷ�Ӧ���Ȼ�ѧ����ʽ______________________��
��5��ʵ���и���60 mL 1.0 mol��L-1�������50 mL 1.1mol��L-1��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������_________�������������������������������к���__________ (��������������������)��
����Ŀ��ʵʩ�Խ�Լ��Դ�ͼ��ٷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����������ѧ֪ʶ���ش��������⣺
��1����֪ij�¶���ij��Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ��K=c(H2O)/[ c(CO)��c(H2)]����Ӧ�Ļ�ѧ��Ӧ����ʽΪ��________________________��
��2����֪��һ���¶��£���C(s)+CO2(g)![]() 2CO(g) ��H1=a kJ/mol ƽ�ⳣ��K1��
2CO(g) ��H1=a kJ/mol ƽ�ⳣ��K1��
��CO(g)+H2O(g)![]() H2(g)+CO2(g) ��H2=b kJ/mol ƽ�ⳣ��K2��
H2(g)+CO2(g) ��H2=b kJ/mol ƽ�ⳣ��K2��
��C(s)+H2O(g)![]() CO(g)+H2(g) ��H3 ƽ�ⳣ��K3��
CO(g)+H2(g) ��H3 ƽ�ⳣ��K3��
��K1��K2��K3֮��Ĺ�ϵ�ǣ�_____________����H3=__________���ú�a��b�Ĵ���ʽ��ʾ����
��3��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���������·�Ӧ��CO(g)+H2O(g)![]() H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
�¶�/�� | 400 | 500 | 800 |
ƽ�ⳣ��K | 9.94 | 9 | 1 |
�÷�Ӧ������Ӧ������_________��Ӧ������ȡ����ȡ���������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020 mol��L-1���ڸ������£�CO��ƽ��ת����Ϊ��______________��
��4���ڴ������������·�Ӧ��H2O(g)+CO(g)![]() CO2(g)+H2(g)��COת������������������ѹǿ�ȼ��¶ȱ仯��ϵ������ͼ��ʾ��
CO2(g)+H2(g)��COת������������������ѹǿ�ȼ��¶ȱ仯��ϵ������ͼ��ʾ��

�������෴Ӧ����ij���(B)��ƽ���ѹǿ(PB)�������ʵ���Ũ��(CB)Ҳ���Ա�ʾƽ�ⳣ��������KP������÷�Ӧ��KP�ı���ʽ��KP=____________�����p[H2O(g)]/p(CO)�ȣ���KP__________����������С�����䡱����ʵ���ϣ���ʹ����þ�����Ĺ�ҵ�����У�һ�����400�����ҡ�p[H2O(g)]/p(CO)=3��5����ԭ�������_________________________________��
��5����ҵ�Ͽ�����ԭ���ԭ����ȥ��ҵβ���е�CO����������ܣ���Ӧװ��������ͼ��ʾ����д�������ĵ缫��Ӧʽ��___________________________________��