ЬтФПФкШн
ЁОЬтФПЁПРћгУЯрЙижЊЪЖЬюПеЁЃ
(1)БъзМзДПіЯТЃЌ11.2LNH3жаКЌ______ИіАБЗжзгЁЃ
(2)ЕШжЪСПЕФSO2КЭSO3ЮяжЪЕФСПжЎБШЪЧ______ЃЛЫљКЌЕФбѕдзгИіЪ§жЎБШЪЧ______ЁЃ
(3)4.8g CH4жаЫљКЌЧтдзгЪ§гы______gЫЎЫљКЌЧтдзгЪ§ЯрЕШЁЃ
(4)24.8gNa2RКЌNa+0.8molЃЌдђNa2RЕФФІЖћжЪСПЮЊ______ЁЃ
(5)БъПіЯТЃЌвЛЖЈСПЕФN2гы22.4LCOЫљКЌЕчзгЕФЮяжЪЕФСПЯрЕШЃЌдђN2ЕФжЪСПЪЧ______ЁЃ
ЁОД№АИЁП0.5NA 5:4 5:6 10.8 62g/mol 28g
ЁОНтЮіЁП
ЃЈ1ЃЉИљОнn=![]() МЦЫуАБЦјЕФЮяжЪЕФСПЃЌНсКЯN=nN AМЦЫуЗжзгЪ§ЃЛ
МЦЫуАБЦјЕФЮяжЪЕФСПЃЌНсКЯN=nN AМЦЫуЗжзгЪ§ЃЛ
ЃЈ2ЃЉИљОнn=![]() =
=![]() НсКЯЗжзгЙЙГЩМЦЫуЃЛ
НсКЯЗжзгЙЙГЩМЦЫуЃЛ
ЃЈ3ЃЉИљОнn=![]() =
=![]() МЦЫуЃЛ
МЦЫуЃЛ
ЃЈ4ЃЉNa +ЮЊ0.8molЃЌдђNa 2XЮЊ0.4molЃЌИљОнM=![]() МЦЫуNa 2XФІЖћжЪСПЃЛ
МЦЫуNa 2XФІЖћжЪСПЃЛ
ЃЈ5ЃЉЕЊЦјКЭCOЗжзгжаЖМКЌга14ИіЕчзгЃЌЖўепКЌгаЕчзгЕФЮяжЪЕФСПЯрЕШЃЌЫЕУїЖўепЮяжЪЕФСПЯрЕШЃЌИљОнn=![]() =
=![]() МЦЫуЁЃ
МЦЫуЁЃ
(1)n=![]() mol=0.5molЃЌКЌга0.5NAИіАБЦјЗжзгЃЌ
mol=0.5molЃЌКЌга0.5NAИіАБЦјЗжзгЃЌ
ЙЪД№АИЮЊЃК0.5NAЃЛ
(2)ЩшжЪСПЖМЮЊmgЃЌдђЕШжЪСПЕФ SO2КЭSO3 ЮяжЪЕФСПжЎБШЪЧ![]() :
:![]() =5:4 ЃЌЫљКЌЕФбѕдзгИіЪ§жЎБШЪЧ 5ЁС2:4ЁС3=5:6 ЃЌ
=5:4 ЃЌЫљКЌЕФбѕдзгИіЪ§жЎБШЪЧ 5ЁС2:4ЁС3=5:6 ЃЌ
ЙЪД№АИЮЊЃК 5:4 ЃЛ 5:6 ЃЛ
(3)n(CH4)=![]() =0.3molЃЌn(H)=1.2molЃЌдђЫЎЕФЮяжЪЕФСПЮЊ 0.6mol ЃЌжЪСПЮЊ 0.6molЁС18g/mol=10.8g ЃЌ
=0.3molЃЌn(H)=1.2molЃЌдђЫЎЕФЮяжЪЕФСПЮЊ 0.6mol ЃЌжЪСПЮЊ 0.6molЁС18g/mol=10.8g ЃЌ
ЙЪД№АИЮЊЃК10.8ЃЛ
(4)Na+ ЮЊ 0.8molЃЌдђ Na2X ЮЊ 0.4molЃЌNa2X ФІЖћжЪСП =![]() =62g/mol ЃЌ
=62g/mol ЃЌ
ЙЪД№АИЮЊЃК62g/mol ЃЛ
(5)БъПіЯТ 22.4L CO ЕФЮяжЪЕФСПЮЊ 1molЃЌЕЊ N2 КЭ CO ЗжзгжаЖМКЌга 14 ИіЕчзгЃЌЖўепКЌгаЕчзгЕФЮяжЪЕФСПЯрЕШЃЌЫЕУїЖўепЮяжЪЕФСПЯрЕШЃЌдђ N2ЕФЮяжЪЕФСПЮЊ 1mol ЃЌЦфжЪСПЮЊЃК 28g/molЁС1mol=28g ЃЌ
ЙЪД№АИЮЊЃК28gЁЃ

ЁОЬтФПЁПфхБНЪЧвЛжжЛЏЙЄдСЯЃЌЪЕбщЪвКЯГЩфхБНЕФзАжУЪОвтЭММАгаЙиЪ§ОнШчЯТЃК
БН | фх | фхБН | |
УмЖШ/gЁЄcm-3 | 0.88 | 3.10 | 1.50 |
ЗаЕу/Ёц | 80 | 59 | 156 |
ЫЎжаШмНтЖШ | ЮЂШм | ЮЂШм | ЮЂШм |
АДЯТСаКЯГЩВНжшЛиД№ЮЪЬтЃК
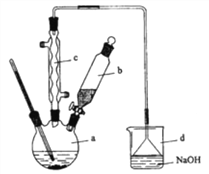
ЃЈ1ЃЉдкaжаМгШы15mLЮоЫЎБНКЭЩйСПЬњаМЁЃдкbжааЁаФМгШы4.0mLвКЬЌфхЁЃЯђaжаЕЮШыМИЕЮфхЃЌгаАзЩЋбЬЮэВњЩњЁЃЧыЗжБ№аДГіaвЧЦїКЭcвЧЦїЕФУћГЦЃК_________________________ЁЂ________________________________ЁЃ
ЃЈ2ЃЉЧыаДГіaжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЃК___________________ЁЃdжаЕЙжУТЉЖЗЕФзїгУЪЧЃК_____ЃЌФГЭЌбЇШЯЮЊШчЙћЯыбщжЄИУЗДгІЕФРраЭЃЌПЩвдШЁЗДгІКѓЩеБdжаШмвКЃЌМгШыЯЁЯѕЫсЫсЛЏЃЌШЛКѓМгШыЯѕЫсвјШмвКЃЌШчЙћВњЩњСЫЕЛЦЩЋГСЕэМДбщжЄСЫИУЗДгІЕФРраЭЁЃЪдЗжЮіИУЭЌбЇЕФВйзїЪЧЗёПЩааЃК______ЃЈЬюЁАЪЧЁБЛђЁАЗёЁБЃЉЃЌМђвЊЫЕУїЦфдвђЃК________________________ЁЃ
ЃЈ3ЃЉвКфхЕЮЭъКѓЃЌОЙ§ЯТСаВНжшЗжРыЬсДПЃК
ЂйЯђaжаМгШы10mLЫЎЃЌШЛКѓЙ§ТЫГ§ШЅЮДЗДгІЕФЬњаМЃЛ
ЂкТЫвКвРДЮгУ10mLЫЎЁЂ8mL10%ЕФNaOHШмвКЁЂ10mLЫЎЯДЕгЁЃ
ЂлЯђЗжГіЕФДжфхБНжаМгШыЩйСПЕФЮоЫЎТШЛЏИЦЃЌОВжУЁЂЙ§ТЫЁЃ
ОвдЩЯЗжРыВйзїКѓЃЌДжфхБНжаЛЙКЌгаЕФжївЊдгжЪЮЊ___ЃЌвЊНјвЛВНЬсДПЃЌЯТСаВйзїжаБиаыЕФЪЧ____ЃЈЬюШые§ШЗбЁЯюЧАЕФзжФИЃЉЃЛ
A.жиНсОЇ B.Й§ТЫ C.еєСѓ D.нЭШЁ
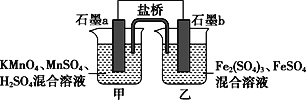
ЁОЬтФПЁПЁАЗЯЦјЁБЕФзлКЯДІРэгыгІгУММЪѕЪЧПЦбаШЫдБЕФживЊбаОППЮЬт,COЁЂSO2ЁЂNO2ЪЧживЊЕФДѓЦјЮлШОЦјЬхЁЃ
ЃЈ1ЃЉДІРэКѓЕФCOЪЧжЦШЁаТаЭФмдДЖўМзУб(CH3OCH3)ЕФдСЯЁЃ
вбжЊЂйCO(g)+H2O(g)![]() CO2(g)+H2(g) ІЄH1=-41.0 kJ/mol
CO2(g)+H2(g) ІЄH1=-41.0 kJ/mol
ЂкCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ІЄH2=-49.0 kJ/mol
CH3OH(g)+H2O(g) ІЄH2=-49.0 kJ/mol
ЂлCH3OCH3(g)+H2O(g)![]() 2CH3OH(g) ІЄH3=+23.5 kJ/mol
2CH3OH(g) ІЄH3=+23.5 kJ/mol
дђЗДгІ2CO(g)+4H2(g)![]() CH3OCH3(g)+H2O(g)ЕФЁїH=________.
CH3OCH3(g)+H2O(g)ЕФЁїH=________.
ЃЈ2ЃЉвбжЊ973 KЪБ,SO: гыNO2 ЗДгІЩњГЩSO,КЭNO,НЋЛьКЯЦјЬхОРфФ§ЗжРыГіЕФSO,ПЩгУгкжЦБИСђЫсЁЃ
Ђй973 KЪБ,ВтЕУ:
NO2(g)![]() NO(g)+ 1/2O2(g) K1=0.018;
NO(g)+ 1/2O2(g) K1=0.018;
SO2(g)+1/2O2(g)![]() SO3(g) K2=20;
SO3(g) K2=20;
дђЗДгІSO2(g)+NO2(g)![]() SO3(g)+NO(g)ЕФK3=________
SO3(g)+NO(g)ЕФK3=________
Ђк973KЪБ,ЯђШнЛ§ЮЊ2 LЕФУмБеШнЦїжаГфШЫSO2ЁЂNO2 Иї0.2molЁЃЦНКтЪБSO2ЕФзЊЛЏТЪЮЊ________ЁЃ
ЂлКубЙЯТ,SO2ЕФЗжбЙPSO2ЫцЮТЖШЕФБфЛЏШчЭМЫљЪО:

ЕБЮТЖШЩ§ИпЪБ,SO2(g)+NO2(g)![]() SO3(g)+NO(g)ЕФЛЏбЇЦНКтГЃЪ§_____(ЬюЁАдіДѓЁБЛђЁАМѕаЁЁБ)ЃЌ ХаЖЯРэгЩ ЪЧ_________.
SO3(g)+NO(g)ЕФЛЏбЇЦНКтГЃЪ§_____(ЬюЁАдіДѓЁБЛђЁАМѕаЁЁБ)ЃЌ ХаЖЯРэгЩ ЪЧ_________.
ЃЈ3ЃЉгУФЩУзЬњПЩШЅГ§ЮлЫЎжаЕФNO3-ЁЃ
ЂйФЩУзЬњЗлгыЫЎжаNO3-ЗДгІЕФРызгЗНГЬЪНЮЊ4Fe+NO3-+10H+=4Fe2++NH4++3H2OЁЃбаОПЗЂЯж,ШєPHЦЋЕЭНЋЛсЕМжТNO3-ЕФШЅГ§ТЪЯТНЕ,ЦфдвђЪЧ___________.
ЂкЯрЭЌЬѕМўЯТ,ФЩУзЬњЗлШЅГ§ВЛЭЌЫЎбљжаЕФNO3-ЕФЫйТЪгаНЯДѓВювьЁЃ
ЯТБэжаЂёКЭЂђВњЩњВювьЕФдвђПЩФмЪЧ____;Ђђжа0~20min,гУNO3-БэЪОЕФЦНОљЗДгІЫйТЪЮЊ____molЁЄL-lЁЄmin-1ЁЃ
ЗДгІЪБМф/min | 0 | 10 | 20 | 30 | 40 | |
Ђё | c(NO3-)/10-4 mol/L | 8 | 3.2 | 1.6 | 0.8 | 0.64 |
Ђђ | c(NO3-)/10-4 mol/L (КЌЩйСПCu2+) | 8 | 0.48 | 0.32 | 0.32 | 0.32 |
ЃЈ4ЃЉгУNaOHШмвКЮќЪеSO2ПЩЕУNaHSO3ШмвК,ЖдNaHSO3ШмвКжаИїРызгХЈЖШЕФЙиЯЕЃЌЯТСаЗжЮіВЛКЯРэЕФЪЧ___ЁЃ(вбжЊГЃЮТЯТK1(H2SO3)=1.5ЁС10-2ЃЌ,K2(H2SO3)=1.02ЁС10-7)
A.[Na+]+[H+]=[HSO3-]+2[SO32-]+[OH-]
B.[Na+]=[HSO3-]+[SO32-]+[H2SO3]
C.[Na+]>[SO32-]>[HSO3-]>[OH-]>[H+]
D.[H+]+[SO32-]=[OH-]+[H2SO3]