��Ŀ����
��1��Pb��NO3��2��Һ�У�
| c(Pb2+) |
| c(NO3-) |
| 1 |
| 2 |
��2����Pb��NO3��2��Һ�еμ�NaOH��Һ����Һ����ǣ���pHԼΪ
��3��ˮ��Ǧ������⣺
������Pb2+Ũ��Ϊ1.0mg/mL�ı���Һ100mL����ȡ
a������ƿ b����ȷ��Ϊ0.001g�ĵ�����ƽ c����ȷ��Ϊ0.1mg�ĵ�����ƽ
�ڽ�������Һϡ��ΪŨ�ȷֱ�Ϊ0.2��1.0��3.0��5.0��7.0��10.0����λ��mg/L������Һ��ϡ��ʱ���ȼ����ᣬȻ���ټ�ˮ������������ԭ����
����������Ƶĸ�Ũ����Һ�м�����ɫ���ֱ�ⶨ��ͬŨ����Һ�Թ�����ճ̶ȣ������ⶨ������Ƴ��������£�
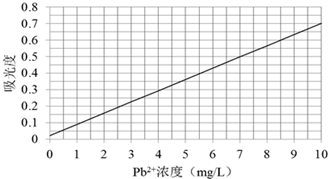
��ȡij������δ�������ķ�ˮˮ��10mL��ϡ����100mL��������۵ķ������вⶨ�����βⶨ���õ���������ݷֱ�Ϊ0.490��0.510����ù�����ˮ������Pb2+Ũ��Ϊ
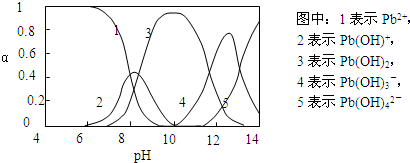
��2����ͼ���Կ�����pH=10ʱ�������3��֪������࣬ΪPb��OH��2�������������������ƣ�������4��5��֪��pH=13ʱ����Pb��OH��3-ת��ΪPb��OH��42-�����ӷ�Ӧ��
��3��������������ҺŨ�Ⱥ���������������ʵ��������Ħ�����������������ʹ�������������������Һ����Ҫ�ľ�ȷ�ȺͲ���ѡ����Ҫ��������
��Pb2+��ˮ��Һ��ˮ�⣬������������ˮ�⣻
����������ȼ���õ���ƽ��ֵ������ͼ��õ�Pb2+��Ũ�ȣ�
| 1 |
| 2 |
�ʴ�Ϊ������
��2����ͼ�е�����3��֪��pH=10ʱ���ɳ�����࣬�������������ƣ�������4��5��֪��pH=13ʱ����Pb��OH��3-ת��ΪPb��OH��42-�����ӷ�Ӧ�����ӷ�ӦΪPb��OH��3-+OH-�TPb��OH��42-��
�ʴ�Ϊ��10��Pb��OH��3-+OH-�TPb��OH��42-��
��3��������Pb2+Ũ��Ϊ1.0mg/mL�ı���Һ100mL����Ҫ����Pb��NO3��2��������Ϊ��
| 1.0mg/ml��100ml��10-3g/mg |
| 207g/mol |
���ƹ����б����õ��Ķ�������������159.9mg����Ҫ��ȷ��Ϊ0.1mg����ƽ������100mL��Һ��Ҫ100mL����ƿ��
�ʴ�Ϊ��159.9mg��ac��
��Pb2+��ˮ��Һ��ˮ�⣬ϡ��ʱ���ȼ����ᣬȻ���ټ�ˮ������������ԭ��������Pb2+��ˮ��Һ�е�ˮ�⣻
�ʴ�Ϊ������Pb2+ˮ�⣻
�ܰ�����۵ķ������вⶨ�����βⶨ���õ���������ݷֱ�Ϊ0.490��0.510��ƽ��Ϊ
| 0.490+0.510 |
| 2 |
�ʴ�Ϊ��70��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����ǵ����Ϻ����ḻ��һ��Ԫ �أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش�
�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش� ��1����һ������ĺ����ܱ������У��������»�ѧ��Ӧ�� N2(g)+3H2(g) 2NH3(g)
��1����һ������ĺ����ܱ������У��������»�ѧ��Ӧ�� N2(g)+3H2(g) 2NH3(g)
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
| t/K | 298 | 398 | 498 | �� |
| K/(mol��L��1)2 | 4.1��106 | K1 | K2 | �� |
 �������⣺
�������⣺�ٱȽ�K1��K2�Ĵ�С��K1 K2���>������=����<������
���жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�������� ������ţ���
A��2v(H2)������=3v(NH3)������ B��v(N2)������=3v(H2)������
C��������ѹǿ���ֲ��� D�����������ܶȱ��ֲ���
��2�������£�N2H6Cl2����һ����Ҫ�Ļ���ԭ�ϣ��������ӻ����������ˮ����Һ�����ԣ�ˮ��ԭ����NH4Cl���ơ�
��д�������µ�һ��ˮ�ⷴӦ�����ӷ���ʽ ��[��Դ:Z_xx_k.Com]
��������ˮ��Һ������Ũ
 �ȵ�����˳����ȷ���� ������ţ���
�ȵ�����˳����ȷ���� ������ţ���A��c(Cl��)>c(N2H62+)>c(H+)>c(OH��)
B��c(Cl��)>c([N2H5��H2O+])> c(H+)>c(OH��)
C��c(N2H62+)+ c([N2H5��H2O+])+c(H+)= c(Cl��)+c(OH��)
D��c(N2H62+)> c(Cl��)>c(H+)>c(OH��)
��ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע�������ϵ�֪Pb4+���к�ǿ�������ԡ�ˮ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb(OH)+��Pb(OH)2����ˮ���ܽ��С����Pb(OH)3����Pb(OH)42��������̬�����ʵ����ķ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ��
 |
��1��Pb(NO3)2��Һ�У�c(Pb2+)/c(NO3��) 1/2�����������=��������������
��2����Pb(NO3)2��Һ�μ����ᣬ��Һ��c(Pb2+)/c(NO3��)û�б������С���г������ɣ������ɵij�������Ϊ ��
��3����Pb(NO3)2��Һ�еμ�NaOH��Һ����ҺҲ����ǣ���pHԼΪ ʱ���ɳ�����࣬�����μ�NaOH��Һ�������ϵ������塣pH = 13ʱ�������ϵ�з�������Ҫ��Ӧ�����ӷ���ʽΪ��
 ��
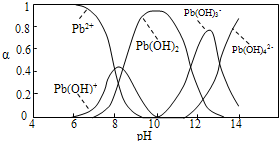
�� ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2����Pb(OH)����Pb(OH)2��Pb(OH)3-��Pb(OH)42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ��

(1)Pb(NO3)2��Һ�У� ________2(�>����������<��)��������Һ�е����Ȼ����Һ��
________2(�>����������<��)��������Һ�е����Ȼ����Һ�� ���ӣ����ܵ�ԭ����________________________________��
���ӣ����ܵ�ԭ����________________________________��
(2)��Pb(NO3)2��Һ�е���ϡNaOH��Һ��pH��8ʱ��Һ�д��ڵ�������(Na������)��__________��pH��9ʱ��Ҫ��Ӧ�����ӷ���ʽΪ_______________________��
(3)ij�������Ʊ���һ��������Ǧ��������Чȥ��ˮ�еĺ���Ǧ��ʵ�������±���
|
���� |
Pb2�� |
Ca2�� |
Fe3�� |
Mn2�� |
Cl�� |
|
����ǰŨ��/(mg��L��1) |
0.100 |
29.8 |
0.120 |
0.087 |
51.9 |
|
������Ũ��/(mg��L��1) |
0.004 |
22.6 |
0.040 |
0.053 |
49.8 |
�ϱ��г�Pb2���⣬����Ǧ�����������ӵ�ȥ��Ч����õ���________��
(4)��� ����Ǧ��(��EH��ʾ)��Ǧ��Ҫ�����ķ�Ӧ������Ϊ��2EH(s)��Pb2�� E2Pb(s)��2H������Ǧ�������pH��ΧΪ(
)
E2Pb(s)��2H������Ǧ�������pH��ΧΪ(
)
A��4��5 B��6��7 C��9��10 D��11��12

 2NH3��g��
2NH3��g��