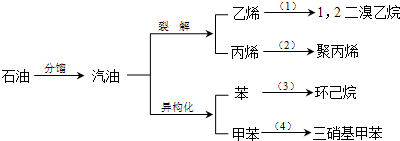
��Ŀ����
����ԭ��Ϊԭ����������ϩ�IJ������£�

��ش�
��1�������������������仯����______������ţ���
��2������ϩ���Ͽ�������������ְ�װ���ϣ�����ϩ�Ľṹ��ʽ��______��
��3����11.2L����״�����������ϩ�Ļ������ͨ����������ˮ�У���ַ�Ӧ����ˮ����������5.6g����ˮ�������ӵ�ԭ���ǣ��û�ѧ����ʽ��ʾ����______��
ԭ��������У���������ϩ�����ʵ���֮����______��
��ʵ��������ͼ��ʾװ����ȡ������������ش�
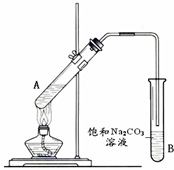
��1�����Թ�A���ȼ�3mL�Ҵ���Ȼ�������2mLŨ�����2mL���ᣬ����֮�䷢����Ӧ�Ļ�ѧ����ʽΪ��
______��
��2������һ��ʱ����Կ����Թ�B��Һ��______����ϡ����¡��������ġ�������ˮ����״Һ��������������ŵ���ζ��
��3��B�Թ��еĵ��ܲ�����Һ���µ�ԭ����______������ţ���
�ٷ�ֹ��Һ�����ڱ��ⷴӦ���죮

��ش�
��1�������������������仯����______������ţ���
��2������ϩ���Ͽ�������������ְ�װ���ϣ�����ϩ�Ľṹ��ʽ��______��
��3����11.2L����״�����������ϩ�Ļ������ͨ����������ˮ�У���ַ�Ӧ����ˮ����������5.6g����ˮ�������ӵ�ԭ���ǣ��û�ѧ����ʽ��ʾ����______��
ԭ��������У���������ϩ�����ʵ���֮����______��
��ʵ��������ͼ��ʾװ����ȡ������������ش�

��1�����Թ�A���ȼ�3mL�Ҵ���Ȼ�������2mLŨ�����2mL���ᣬ����֮�䷢����Ӧ�Ļ�ѧ����ʽΪ��
______��
��2������һ��ʱ����Կ����Թ�B��Һ��______����ϡ����¡��������ġ�������ˮ����״Һ��������������ŵ���ζ��
��3��B�Թ��еĵ��ܲ�����Һ���µ�ԭ����______������ţ���
�ٷ�ֹ��Һ�����ڱ��ⷴӦ���죮
��1�����������û�����������ɣ����������仯���ʴ�Ϊ���٣�
��2������ϩ�Ľṹ��ʽ��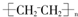 ���ʴ�Ϊ��
���ʴ�Ϊ��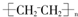 ��
��
��3����ϩ����˫����������ˮ�����ӳɷ�Ӧ��CH2=CH2+Br2��CH2BrCH2Br��11.2L�����������ʵ���Ϊn=
=
=0.5mol��
��ϩ����˫����������ˮ�����ӳɷ�Ӧ����ϩ������Ļ������ͨ��������ˮ�У���ַ�Ӧ����ˮ������������5.6g����Ϊ��ϩ��������
������ϩ�����ʵ���Ϊn=
=
=0.2mol��
����������ʵ���Ϊ��0.5mol-0.2mol=0.3mol������Ϊ��0.3mol��30g/mol=9g��
���ԣ���ϩ����������ʵ���֮��Ϊ0.2mol��0.3mol=2��3���ʴ�Ϊ��CH2=CH2+Br2��CH2BrCH2Br��3��2��
��1���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪ
CH3COOH+CH3CH2OH
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+HOCH2CH3
CH3COOCH2CH3+H2O��
��2��CH3COOC2H5���ܶȱ�ˮ��С������ˮ�����棬�ʴ�Ϊ���ϣ�
��3���Ҵ�������������ˮ��������Һ�����£����ܷ����������ʴ�Ϊ���٣�
��2������ϩ�Ľṹ��ʽ��
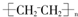 ���ʴ�Ϊ��
���ʴ�Ϊ��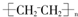 ��
����3����ϩ����˫����������ˮ�����ӳɷ�Ӧ��CH2=CH2+Br2��CH2BrCH2Br��11.2L�����������ʵ���Ϊn=
| V |
| Vm |
| 11.2L |
| 22.4L/mol |
��ϩ����˫����������ˮ�����ӳɷ�Ӧ����ϩ������Ļ������ͨ��������ˮ�У���ַ�Ӧ����ˮ������������5.6g����Ϊ��ϩ��������
������ϩ�����ʵ���Ϊn=
| m |
| M |
| 5.6g |
| 28g/mol |
����������ʵ���Ϊ��0.5mol-0.2mol=0.3mol������Ϊ��0.3mol��30g/mol=9g��
���ԣ���ϩ����������ʵ���֮��Ϊ0.2mol��0.3mol=2��3���ʴ�Ϊ��CH2=CH2+Br2��CH2BrCH2Br��3��2��
��1���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪ
CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
| ||
| �� |
��2��CH3COOC2H5���ܶȱ�ˮ��С������ˮ�����棬�ʴ�Ϊ���ϣ�
��3���Ҵ�������������ˮ��������Һ�����£����ܷ����������ʴ�Ϊ���٣�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ