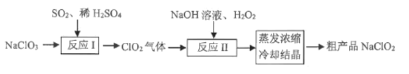
��Ŀ����
����Ŀ����ͼ��ʾ���Ժ�ˮΪԭ�ϣ����ij����Ĺ�������ͼ����֪���������У���Һ��pH������С���Իش��������⣺

��1��ͼ�������Ȼ��������Ƿ���ͬ________(ѡ��ǡ���)��
��2���������з�Ӧ�Ļ�ѧ����ʽΪ_______________________________����2���Ȼ������ӷ���ʽΪ_________________________________________________��
��3������֤ʵ�������Խ����У���1���Ȼ������п���������������������ʵ�ʹ�ҵ������Ϊʲô���ø����۵Ŀ����������________________________��
��4�������յĴ��������ɼ���Ϊ������������X�������ա��Ȼ�����ijͬѧ��Ϊ�ڹ�ҵ���������жԵ�1���Ȼ�Һֱ������Ҳ�ɵõ�������ؽ����������̣���Ը�ͬѧ��˵��������ȷ������___________________________��
���𰸡��� Br2��SO2��2H2O��H2SO4��2HBr 2Br����Cl2��Br2��2Cl�� �������۵Ŀ������Cl2���е�1���Ȼ�����Ӧ�����ӷ���ʽΪ4Br����O2��4H����2Br2��2H2O����Ӧʱ��Ҫ����������±(Ũ�Ȳ���)�ữ��Ӧ����Ҫ����Һ�кͲ����ŷţ��ھ����ϲ����� ��һ���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��ߣ�������������SO2���ա��Ȼ����Ĺ���ʵ������һ����ĸ������̣��������Ũ�ȣ�������Դ������
��������
�����������Ǻ�ˮ��Br����Cl2������Ȼ�����ȿ�������������������Ϣ������������ҺpH������С����ȷ������X��SO2���������ڷ�Ӧ�Ļ�ѧ����ʽΪBr2��SO2��2H2O��H2SO4��2HBr�����ɵ�Br���ٱ�Cl2���������ɵ�Br2�������������������õ�Һ̬�塣
��1��ͼ�������Ȼ���������ͬ�������������������ã�
��2���������з�Ӧ�Ļ�ѧ����ʽΪBr2��SO2��2H2O��H2SO4��2HBr����2���Ȼ������ӷ���ʽΪ2Br����Cl2��Br2��2Cl����
��3���������۵Ŀ������Cl2���е�1���Ȼ�����Ӧ�����ӷ���ʽΪ4Br����O2��4H����2Br2��2H2O����Ӧʱ��Ҫ����������±(Ũ�Ȳ���)�ữ��Ӧ����Ҫ����Һ�кͲ����ŷţ��ھ����ϲ����㣬�����ʵ�ʹ�ҵ�����в��ø����۵Ŀ������������
��4����һ���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��ߣ�������������SO2���ա��Ȼ����Ĺ���ʵ������һ����ĸ������̣��������Ũ�ȣ�������Դ�����ġ�

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�����Ŀ��ʵ���ҿ��������������Ʊ���ˮ���Ȼ�����SnCl4����SnCl4�ӷ���������ˮ�⣬Cl2��������SnCl4���Ʊ�ԭ����ʵ��װ��ͼ��ͼ��
Sn(s)+2Cl2(g)=SnCl4(l) ��H=�C511kJmol-1
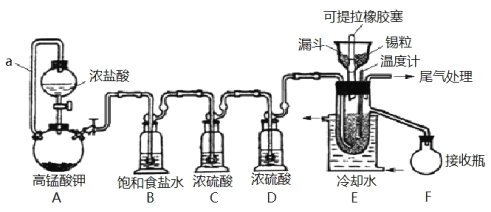
�����õ����й��������£�
���� | Sn | SnCl4 | CuCl2 |
�۵�/�� | 232 | -33 | 620 |
�е�/�� | 2260 | 114 | 993 |
�Ʊ������У����������ģ�������������ʱ��Ӧ���в�����������SnCl4Һ��������ڸ߶�ʱ��Һ̬���ᆳ����������ƿ���ش��������⣺
��1��a��������___��
��2��A�з�Ӧ�Ļ�ѧ����ʽ��___��
��3��B��������___��
��4��E����ȴˮ��������___��
��5��β������ʱ����ѡ�õ�װ����___������ţ���
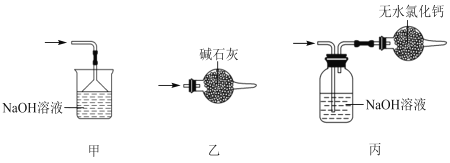
��6�������к�ͭ������ E �в��� CuCl2������Ӱ�� F �в�Ʒ�Ĵ��ȣ�ԭ����___��
��7��SnCl4��Ʒ�к���Cl2������ʱ����������м������ɵô�����SnCl4����������в���Ҫ�õ���������___(�����)
A����Һ©�� B���¶ȼ� C������ƿ D�������� E��������ƿ
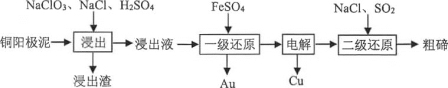
����Ŀ��������Ϊ�����Դ���Ź㷺��Ӧ��ǰ����������Ȼ���Ʊ��������������¡�

��ش��������⣺
I��ת��������Ȼ��ѹ����������30��ʱ����T��F�������£����Ի������������ʾ��ͼ���¡�
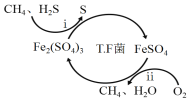
(1)����i�����ӷ�Ӧ����ʽΪ_________________________________________��
(2)��֪��
��Fe3+��pH=l.9ʱ��ʼ������pH=3.2ʱ������ȫ��
��30��ʱ����T.F�������£���ͬpH��FeSO4��Һ��Fe2+�������������±���
pH | 0.7 | 1.1 | 1.5 | 1.9 | 2.3 | 2.7 |
Fe2+����������/g��L-1��h-1 | 4.5 | 5.3 | 6.2 | 6.8 | 7.0 | 6.6 |
��ת�������У������ϱ���ѡ�����pH��Χ��_______<pH<_______������ѡ���ԭ���ǣ�_______________________________________________��
������ת�����ڴ����������£�ˮ������CH4�����������ͼ�ش����⡣
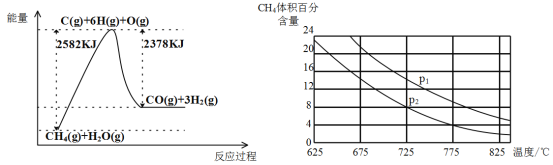
(3)�ٸù��̵��Ȼ�ѧ����ʽ��__________________________________________��
�ڱȽ�ѹǿP1��p2�Ĵ�С��ϵ��P1 _________ P2(ѡ����>����<������=��)��
����һ���¶Ⱥ�һ��ѹǿ�µ�����ɱ���ܱ������г���1molCH4��1mol��ˮ������ַ�Ӧ��ƽ������ʼʱ��������ܶ���ƽ��ʱ������ܶȵ�1.4��������ʱ���������Ϊ2L,��÷�Ӧ��ƽ�ⳣ��Ϊ______________(�������2λ��Ч����)��
����CO�任��500��ʱ��CO��һ����ˮ��Ӧ����CO2��H2��
����H2�ᴿ����CO2��H2����õ�H2�Ĺ�����ʾ��ͼ
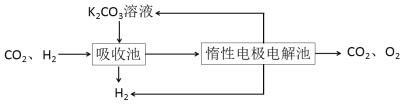
(4)���ճ��з�����Ӧ�����ӷ���ʽ��____________________________________��