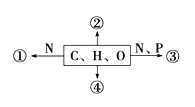
��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣������г�11��Ԫ�������ڱ��е�λ�ã���Ҫ��ش����и��⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� | �� |
��1����11��Ԫ���У���ѧ��������õ�Ԫ����______����Ԫ�ط��ţ���ͬ�����õ���������ǿ��ԭ����______��ʧ����������ǿ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ��________________________�������µ���ΪҺ̬�ķǽ���������____________��
��2��Ԫ�آܵ����ӽṹʾ��ͼΪ__________________��
��3���ٺ͢�����Ԫ������������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ___________________��
�ں͢�����Ԫ������������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ___________________��
�ڵ�����������Ӧˮ�����ˮ��Һ��ݵ���������ﷴӦ�Ļ�ѧ����ʽΪ_________��
���𰸡� Ne F 2K+2H2O=====2KOH+H2�� Br2 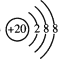 Al(OH)3+OH- ===== AlO2-+2H2O H++OH- ===== H2O Al2O3+2OH- ===== 2AlO2-+H2O
Al(OH)3+OH- ===== AlO2-+2H2O H++OH- ===== H2O Al2O3+2OH- ===== 2AlO2-+H2O
������������Ԫ����Ԫ�����ڱ��е�λ�ÿ���֪��,��ΪNa,��ΪK,��ΪMg,��ΪCa,��ΪAl,��ΪC,��ΪP,��ΪF,��ΪCl,��ΪBr, ΪNe
(1)����Ԫ����ֻ��Ne����������Ϊ8,Ϊ�ȶ��ṹ,��ѧ��������õ�Ԫ��Ne,����Ԫ����ֻ��F�ķǽ�������ǿ,��![]() �õ���������ǿ,ֻ��K�Ľ�������ǿ,��ˮ��Ӧ����,�÷�ӦΪ2K+2H2O=2KOH+H2�� ,�嵥���ڳ�����ΪҺ��,�仯ѧʽΪBr2,��ˣ�������ȷ����:Ne;F2; 2K+2H2O=2KOH+H2��; Br2;
�õ���������ǿ,ֻ��K�Ľ�������ǿ,��ˮ��Ӧ����,�÷�ӦΪ2K+2H2O=2KOH+H2�� ,�嵥���ڳ�����ΪҺ��,�仯ѧʽΪBr2,��ˣ�������ȷ����:Ne;F2; 2K+2H2O=2KOH+H2��; Br2;
(2)Ca��������Ϊ20,��ʧȥ�����2�����ӱ�Ϊ������,���ӽṹʾ��ͼΪ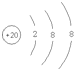 ,��ˣ�������ȷ����:
,��ˣ�������ȷ����: ��
��
(3) ��ΪNa�͢�ΪAl������Ԫ������������Ӧ��ˮ����ֱ�ΪNaOH�� Al(OH)3���������Ӧ�����ӷ���ʽΪAl(OH)3+OH- ===== AlO2-+2H2O��
��ΪK�͢�,��ΪCl������Ԫ������������Ӧ��ˮ����ֱ�ΪKOH��HClO4.,���ڿ�����ǿ���ǿ��������Ӧ�����ӷ���ʽΪH++OH- = H2O��
��ΪK������������Ӧˮ����ΪKOH����ΪAl�����������ΪAl2O3����Ӧ�Ļ�ѧ����ʽΪAl2O3+2OH-=2AlO2-+H2O��