��Ŀ����
�Ƽ��仯������й㷺����;��
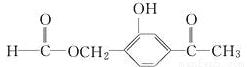
��1����ҵ���Ʊ������Ƶij��÷�����_______����д���Ʊ������ƵĻ�ѧ����ʽ_____________�������ƿ�����________________��д��Na���۵�ͷ����һ����;����
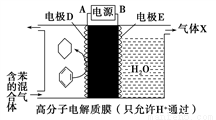
��2����Na2CO3������������ʣ�CO��O2��CO2Ϊԭ�Ͽ�������͵�ء��õ�صĽṹ��ͼ��ʾ��

�������ĵ缫��ӦʽΪ_______����ع���ʱ����A��ѭ��ʹ�ã�A���ʵĻ�ѧʽΪ_______��
����д������Na2CO3����Ԫ�صķ���_________________________��
��3�������£�Ũ�Ⱦ�Ϊ0.1 mol��L��1����������������Һ��pH���±���
���� | CH3COONa | Na2CO3 | NaClO | NaCN |
pH | 8.8 | 11.6 | 10.3 | 11.1 |
��������Һ���������У����H��������ǿ����_________�����ݱ������ݣ�Ũ�Ⱦ�Ϊ0.01 mol��L��1���������������Һ�ֱ�ϡ��100����pH�仯������_______������ţ���
a��HCN b��HClO c��CH3COOH d��H2CO3
��4��ʵ�����г���NaOH������β��������ϴ�����ᴿ��
�ٳ����£���300 mL 1 mol��L��1��NaOH��Һ����4.48 L������ɱ�״����SO2ʱ��������ҺpH>7������Һ�и�����Ũ���ɴ�С��˳��Ϊ_______��
����֪�������ӿ�ʼ����ʱ��pH���±���
���� | Fe2�� | Cu2�� | Mg2�� |
pH | 7.6 | 5.2 | 10.4 |
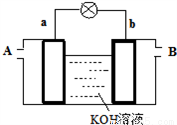
������ͬŨ��Cu2����Mg2����Fe2������Һ�еμ�ijŨ�ȵ�NaOH��Һʱ��_______�������ӷ��ţ��ȳ�����Ksp_______Ksp�����������������������
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�