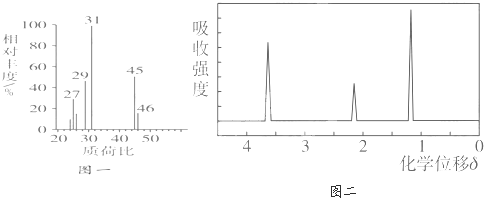
��Ŀ����
��. ��6�֣��л���A�Ľṹ��ʽ��ͼ��ʾ��

��1��A�����NaOH��ȫ��Ӧʱ��A��μӷ�Ӧ��NaOH�����ʵ���֮��Ϊ_________________��
��2��A������Cu��OH��2��ȫ��Ӧʱ������A�뱻��ԭ��Cu��OH��2�����ʵ���֮��Ϊ_________________��
��3��A�������NaHCO3��ȫ��Ӧʱ��A��μӷ�Ӧ��NaHCO3���ʵ���֮��Ϊ_________________��
��. ��14�֣�����ϩΪԭ�Ͽ���ͨ����ͼ��ʾ·�ߺϳ�E��H�����ַ�Ӧ��������ȥ����

��1��A�к��еĹ���������Ϊ_________________��
��2��G�����ᷴӦ����H�ķ�Ӧ����Ϊ_________________��Cת��ΪD�ķ�Ӧ������_________________��
��3��д��D������NaOH��Һ��ȫ��Ӧ�Ļ�ѧ����ʽ_________________��
��4��F�Ľṹ��ʽΪ_________________��H�Ľṹ��ʽΪ_________________��
��5��д��ͬʱ��������������B��һ��ͬ���칹��Ľṹ��ʽ_________________��
A. �ܷ���������Ӧ
B. �˴Ź�������ֻ��4����
C. ����FeCl3��Һ������ɫ��Ӧ��ˮ��ʱÿmol������3molNaOH
��. ��6��ÿ��2�֣���1��1��5 ��2��1��2 ��3��1��1
��. ��ÿ��2�֣���14�֣���1��ȩ�� ��2����������ȡ������Ӧ��ȡ����Ӧ��
![]()

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� ������ͬ���칹���к��б����ṹ�Ĺ��У������������칹����������
������ͬ���칹���к��б����ṹ�Ĺ��У������������칹����������| A��1�� | B��3�� | C��4�� | D��5�� |
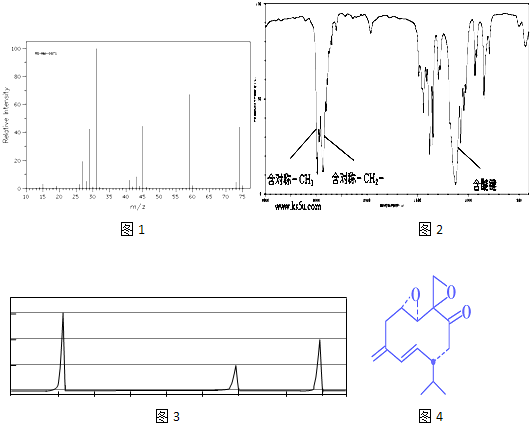

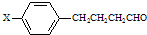 ��X���ܵĽṹ��
��X���ܵĽṹ��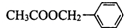 �������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·���磺
�������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·���磺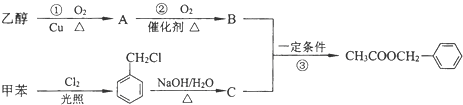


 �л���A�Ľṹ��ʽ��ͼ��ʾ��
�л���A�Ľṹ��ʽ��ͼ��ʾ��