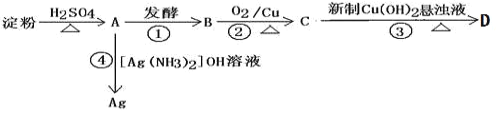
��Ŀ����
����Ŀ��A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ�����������������У�A��C��B��D�ֱ���ͬ����Ԫ�أ�AԪ�ص�ԭ�Ӱ뾶����������Ԫ����ԭ�Ӱ뾶��С�ģ�B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵�2��������Ԫ�����γɵĵ�����A��B���������壬C��D�����ǹ��塣
(1)д������Ԫ�ص����ƣ�B________________��C______________��
(2)д��DԪ�������ڱ��е�λ��___________________________
(3)�õ���ʽ��ʾC2D���γɹ��̣�_______________________________
(4)д����B��C��Ԫ�����γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵĵ���ʽ_______������_______(��������������������)��������ڵĻ�ѧ����������______________________��д������ˮ��Ӧ�����ӷ���ʽ_______________________________
���𰸡��� �� �������ڣ���A�� ![]()
![]() ���� ���Ӽ����Ǽ��Լ�(���ۼ�) 2Na2O2+2H2O=4Na��+4OH��+O2��
���� ���Ӽ����Ǽ��Լ�(���ۼ�) 2Na2O2+2H2O=4Na��+4OH��+O2��
��������
A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶����������Ԫ����ԭ�Ӱ뾶��С�ģ�A��Cͬ���壬��AΪHԪ�أ�B��Dͬ���壬�ֱ��ڶ��������ڣ�����������֮������Ϊ11����Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵Ķ�����C������ΪLi������֪CΪNa����B��D������֮��Ϊ(1+11)��2=24����B��������Ϊx����D��������Ϊx+8����x+x+8=24�����x=8����BΪOԪ�ء�DΪSԪ�أ��ݴ���գ�
(1)�����Ϸ�����BΪ����CΪ�ƣ�
��Ϊ�������ƣ�
(2)д��DΪ��Ԫ�أ��������ڱ��е�λ��Ϊ�������ڣ���A�壻
��Ϊ���������ڣ���A�壻
(3) C2D�����ƣ����õ���ʽ��ʾC2D���γɹ���Ϊ��![]() ��
��
����![]() ��
��
(4)��B��C��Ԫ�����γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����Ϊ�������ƣ������ʽΪ![]() �����������������ӻ���������Ӻ�
�����������������ӻ���������Ӻ�![]() ֮�������Ӽ���
֮�������Ӽ���![]() �ڴ����зǼ��Լ�(���ۼ�)������������ˮ��Ӧ�����������ƺ������������ӷ���ʽΪ��2Na2O2+2H2O=4Na��+4OH��+O2����
�ڴ����зǼ��Լ�(���ۼ�)������������ˮ��Ӧ�����������ƺ������������ӷ���ʽΪ��2Na2O2+2H2O=4Na��+4OH��+O2����
��Ϊ��![]() �����ӣ����Ӽ����Ǽ��Լ�(���ۼ�)��2Na2O2+2H2O=4Na��+4OH��+O2����
�����ӣ����Ӽ����Ǽ��Լ�(���ۼ�)��2Na2O2+2H2O=4Na��+4OH��+O2����

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�����Ŀ������ʵ���Ӧ�������д�����ǣ� ��
ѡ�� | ʵ�� | ���� | ���� |
A | ��AgCl����Һ�м���KI��Һ���� | �����ɰ�ɫ��Ϊ��ɫ | AgCl�ܽ�ȴ���AgI |
B | ��2mL0.1mol/L��FeCl3��Һ�м��������ۣ�����1��KSCN��Һ | ��ɫ����ʧ����KSCN��Һ��ɫ���� | ��ԭ�ԣ�Fe>Fe2+ |
C | �����£�����������Ũ�ȵ�NaHCO3��CH3COONa��Һ�зֱ�μ�2�η�̪ | ������Һ����죬NaHCO3��Һ��ɫ���� | �����µ�ˮ��ƽ�ⳣ���� Kh(CH3COO-)��Kh(HCO3-) |
D | ����ʱ������֧�Թܸ�ȡ4mL 0.1mol/L ����KMnO4��Һ���ֱ����0.1mol/L��0.2 mol/L H2C2O4��Һ��2 mL | ���Թ���Һ����ɫ���Ҽ�0.2mol/L H2C2O4 ��Һ���Թ�����ɫ���� | �����������䣬H2C2O4��Һ��Ũ��Խ��ѧ��Ӧ����Խ�� |
A. AB. BC. CD. D