��Ŀ����
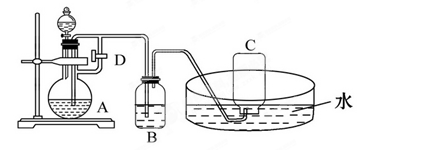
��12�֣���ͼ��ʾװ�ÿ�������ȡ�۲�Fe(OH)2�ڿ����б�����ʱ����ɫ�仯��ʵ��ʱ����ʹ����м��6 mol/L����������Լ���ѡ��
��д���пհף�
��1��B��ʢ��һ������NaOH��Һ��A�� ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽΪ____________________________________________________��
ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽΪ____________________________________________________��
��2��ʵ�鿪ʼʱӦ�Ƚ�����D_____����Ŀ����____����C���ռ�����������Ҫ��_____________________
��3����������Fe(OH)2�IJ�������______________________________________________
��4���ε�װ��B�е�������ʹ�������룬д���йط�Ӧ�Ļ�ѧ����ʽ________________

��д���пհף�
��1��B��ʢ��һ������NaOH��Һ��A��
 ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽΪ____________________________________________________��
ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽΪ____________________________________________________����2��ʵ�鿪ʼʱӦ�Ƚ�����D_____����Ŀ����____����C���ռ�����������Ҫ��_____________________
��3����������Fe(OH)2�IJ�������______________________________________________
��4���ε�װ��B�е�������ʹ�������룬д���йط�Ӧ�Ļ�ѧ����ʽ________________
��1����м Fe+2H+==Fe2++H2��
��2�� �� �ų�B�еĿ��� ����
��3���رջ���D��ʹA�е�FeSO4��Һѹ��Bƿ����NaOH��Ӧ���Ӷ��Ƶ�Fe(OH)2
��4��4Fe(OH)2+O2+2H2O====4Fe(OH)3

��2�� �� �ų�B�еĿ��� ����
��3���رջ���D��ʹA�е�FeSO4��Һѹ��Bƿ����NaOH��Ӧ���Ӷ��Ƶ�Fe(OH)2
��4��4Fe(OH)2+O2+2H2O====4Fe(OH)3
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CuO
CuO Cu(NO3)2
Cu(NO3)2  H2O��
H2O��