��Ŀ����
ʵ�����ﳣ�á��ⴸ���IJ����������������ʵ�飬������Ϊ��ͷ�ܷ�IJ� �������м����һ�������̱����ô����������й�ʵ��ʱ������װ�ü��������㡢�������ԡ��ɷ���ʹ�õ��ŵ㡣�ô��������ܷ������е�ʵ����
�������м����һ�������̱����ô����������й�ʵ��ʱ������װ�ü��������㡢�������ԡ��ɷ���ʹ�õ��ŵ㡣�ô��������ܷ������е�ʵ����
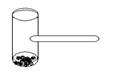
 �������м����һ�������̱����ô����������й�ʵ��ʱ������װ�ü��������㡢�������ԡ��ɷ���ʹ�õ��ŵ㡣�ô��������ܷ������е�ʵ����
�������м����һ�������̱����ô����������й�ʵ��ʱ������װ�ü��������㡢�������ԡ��ɷ���ʹ�õ��ŵ㡣�ô��������ܷ������е�ʵ����
| A��NH4Cl���ȷֽ��ʵ�� |
| B����ˮCuSO4�뵨���Ļ���ʵ�� |
| C�����ͺ��ף��Ѿ����O2����һ���¶����ת���ʵ�� |
| D��KMnO4���ȷֽ��ʵ�� |
D
��

��ϰ��ϵ�д�
�����Ŀ
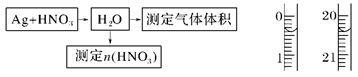
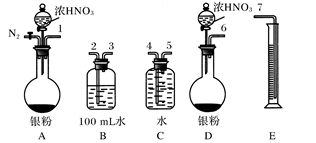
 [Cu(NH3)3]Ac��CO + Q
[Cu(NH3)3]Ac��CO + Q NO����ʵ������ͼ���£�
NO����ʵ������ͼ���£�
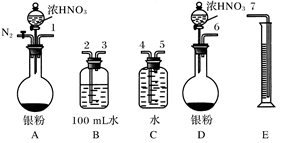
 ����ͭ��������Ӧ����CuO��CuO����ϡ���ᷴӦ�������й���������ȷ����
����ͭ��������Ӧ����CuO��CuO����ϡ���ᷴӦ�������й���������ȷ����