��Ŀ����
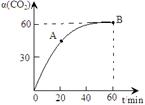
���������������˹��ϳɵ��л����ҵ����������CO2��NH3��һ�������ºϳɣ��䷴Ӧ����ʽΪ2NH3+CO2 CO(NH2)2+H2O������̼��
CO(NH2)2+H2O������̼��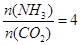 ��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ������˵��������ǣ� ��
��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ������˵��������ǣ� ��
 CO(NH2)2+H2O������̼��
CO(NH2)2+H2O������̼�� ��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ������˵��������ǣ� ��
��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ������˵��������ǣ� ��
| A���÷�Ӧ��60minʱ�ﵽƽ��״̬ |
| B��NH3��ƽ��ת����Ϊ30% |
| C�����Ӱ�̼�ȿɽ�һ�����CO2��ƽ��ת���� |
| D��A����淴Ӧ���ʦ���(CO2)����B�������Ӧ���ʦ���(CO2) |
D
�����������ͼ���֪60minʱ�ﵽƽ��״̬��������̼��ת����Ϊ60%����������ת����Ϊ30%��D��A��δ��ƽ�⣬����CO2������Ӧ���ʴ����淴Ӧ���ʡ�

��ϰ��ϵ�д�
�����Ŀ
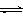 2Z(g)
2Z(g)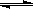 3 C(g)�ﵽƽ��ʱ�����c(A)��0.5 mol/L�����¶Ȳ��������£��������������һ�������ﵽ�µ�ƽ��ʱ�����c(A)��0.20mol/L�������ж�����ȷ����
3 C(g)�ﵽƽ��ʱ�����c(A)��0.5 mol/L�����¶Ȳ��������£��������������һ�������ﵽ�µ�ƽ��ʱ�����c(A)��0.20mol/L�������ж�����ȷ���� 2CrO42-��2H+����K2Cr2O7����ˮ���ϡ��Һ�dz�ɫ��
2CrO42-��2H+����K2Cr2O7����ˮ���ϡ��Һ�dz�ɫ��


 2SO3�ﵽһ���Ⱥ�ͨ��18O2,һ��ʱ��18O���ܴ����ڣ� ��
2SO3�ﵽһ���Ⱥ�ͨ��18O2,һ��ʱ��18O���ܴ����ڣ� �� 2C��g��һ��ʱ���ﵽƽ�⣬����n mol C��������˵���в���ȷ���ǣ� ��
2C��g��һ��ʱ���ﵽƽ�⣬����n mol C��������˵���в���ȷ���ǣ� ��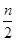 ��
�� 2C(g) ��H=��QkJ/mol���мס��������ݻ���ͬ�Ҳ�����ܱ���������������м���1 mol A��3 mol B����һ�������´ﵽƽ��ʱ�ų�����ΪQ1kJ������ͬ�������£����������м���2 mol C�ﵽƽ�����������ΪQ2 kJ����֪Q1=3Q2��������������ȷ���ǣ� ��
2C(g) ��H=��QkJ/mol���мס��������ݻ���ͬ�Ҳ�����ܱ���������������м���1 mol A��3 mol B����һ�������´ﵽƽ��ʱ�ų�����ΪQ1kJ������ͬ�������£����������м���2 mol C�ﵽƽ�����������ΪQ2 kJ����֪Q1=3Q2��������������ȷ���ǣ� ��

 H2(g)+ CO(g)
H2(g)+ CO(g) 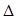 H>0�������ݻ��̶�Ϊ2L�ܱ������У�����2mol C(s)��2mol H2O(g)����T���³�ַ�Ӧ������Ӻ�ﵽƽ�⣬���H2Ϊ0.75mol��
H>0�������ݻ��̶�Ϊ2L�ܱ������У�����2mol C(s)��2mol H2O(g)����T���³�ַ�Ӧ������Ӻ�ﵽƽ�⣬���H2Ϊ0.75mol��