��Ŀ����
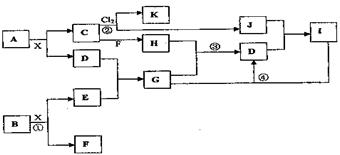
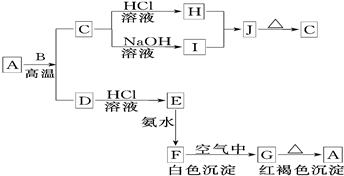
�������й����ʵ�ת����ϵͼ(��Щ���ʼ�ʡ��)������AΪ���ʣ�E�ڳ�����ΪҺ�壬D��һ�ֺ����Ļ������ش�������⡣
(1)��C��ʽ��Ϊ78�����C�Ľṹ�������ƶ��в���ȷ����_____
A�������ڿ����л��ɰ�ɫ
B������ǿ��������
C������������Ӽ���Ǽ��Լ�
D����ʪ�����ɫʯ����ֽ�Ӵ�ʱ��ֻ��ʹ��ֽ����ɫ
(2)A��ԭ�ӽṹ��ͼ______________��H�ĵ���ʽ___________��E�Ľṹʽ_________
(3)��CҲ�Ǻ�������������Ϊ18Oʱ��д��C��D��Ӧ�ķ���ʽ___________________
_______________����һ����(D����)��Ħ������Ϊ______��
(4)��A������ʱ��I��������____________
A��H2O B��NaCl(aq) C��NaOH(aq) D��CuCl2
(5)��û��E�ڳ�����Ϊ��ɫҺ������ƣ���E��I���ɷֱ���_________��_________(����������)

(1)��C��ʽ��Ϊ78�����C�Ľṹ�������ƶ��в���ȷ����_____
A�������ڿ����л��ɰ�ɫ
B������ǿ��������
C������������Ӽ���Ǽ��Լ�
D����ʪ�����ɫʯ����ֽ�Ӵ�ʱ��ֻ��ʹ��ֽ����ɫ
(2)A��ԭ�ӽṹ��ͼ______________��H�ĵ���ʽ___________��E�Ľṹʽ_________
(3)��CҲ�Ǻ�������������Ϊ18Oʱ��д��C��D��Ӧ�ķ���ʽ___________________
_______________����һ����(D����)��Ħ������Ϊ______��
(4)��A������ʱ��I��������____________
A��H2O B��NaCl(aq) C��NaOH(aq) D��CuCl2
(5)��û��E�ڳ�����Ϊ��ɫҺ������ƣ���E��I���ɷֱ���_________��_________(����������)
(1) D(2��) (2)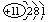 ��
��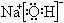 H-O-H��
H-O-H��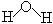 (3��)
(3��)
(3)2Na218O2+2CO2=2Na2CO2��18O+18O2��36g/mol��(3��)
(4)D (2��) (5)�����SO3������������(2��)
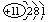 ��
��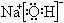 H-O-H��
H-O-H�� (3��)
(3��)(3)2Na218O2+2CO2=2Na2CO2��18O+18O2��36g/mol��(3��)
(4)D (2��) (5)�����SO3������������(2��)
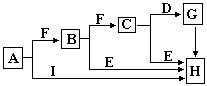
����A����ͬһ����F�����������η�Ӧ�������ĵ��ʳ�������C��S��N2(��ʱFΪO2)��AΪP��FΪCl2�����C��ʽ��ֵ�ɳ���ȷ��AΪ�ơ�CΪNa2O2��BΪNa2O��DΪCO2��GΪNa2CO3��H��NaOH����ʱI��������CuCl2��Na2O2�ڿ����л���CO2��Ӧ����ת��ΪNa2CO3��ɫ���壬�����д����������Ӽ��롰O-O�����ۼ������е���Ԫ��Ϊ-1�ۣ�����ǿ�������ԣ���Ư�װ������ָʾ�����ڵ�������ɫ���ʣ�Ҳ�ܱ��ֳ�һ���Ļ�ԭ�ԣ���ˮ��CO2��Ӧ��һ������������ԭ��Ӧ����EΪ�����SO3��IΪ����������ʱҲ��ʵ�������仯��

��ϰ��ϵ�д�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ