��Ŀ����
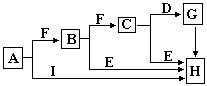
ij��ѧ��ȤС���ú�A��B���ֽ������ʵķ�ĩ״������������ʵ�飬��ת����ϵ��ͼ��ʾ�����ַ�Ӧ���������δ�г���������A��B���ֽ������ʳ�������ŨH2SO4��������γ�һ�����ܵ������ﱡĤ��EΪ��ɫ������IΪ���ɫ������
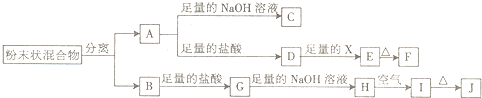
��1��д���������ʵĻ�ѧʽ��F______��G______��
��2������������ķ�����______��
��3��D��Eת���У�����������X���Լ�X������______��
A������NaCl��ҺB��NaOH��ҺC����ˮD��Ba��OH��2��Һ
��4��д������ת���Ļ�ѧ����ʽ��A��C��______��H��I��______��

��1��д���������ʵĻ�ѧʽ��F______��G______��
��2������������ķ�����______��
��3��D��Eת���У�����������X���Լ�X������______��
A������NaCl��ҺB��NaOH��ҺC����ˮD��Ba��OH��2��Һ
��4��д������ת���Ļ�ѧ����ʽ��A��C��______��H��I��______��
A��B���ֽ������ʵķ�ĩ״��������A��B���ֽ������ʳ�������ŨH2SO4��������γ�һ�����ܵ������ﱡĤ���ж�ΪFe��Al��ת����ϵ�з�����֪A���ᡢ�Ӧ�ж�AΪAl��BΪFe����CΪNaAlO2��DΪAlCl3��EΪ��ɫ����˵������������������֤��XΪ����Һ��FΪAl2O3��IΪ���ɫ����ΪFe��OH��3��HΪFe��OH��2��JΪFe2O3��GΪFeCl2��
��1�����������ж�FΪ����������ѧʽΪAl2O3��GΪ�Ȼ���������ѧʽFeCl2��
�ʴ�Ϊ��Al2O3��FeCl2��
��2��������������ķ������ô�������������
�ʴ�Ϊ���ô���������ĩ״���������������ڴ����ı��棻
��3��DΪAlCl3��EΪ��ɫ����˵������������������֤��XΪ����Һ��D��Eת���У�����������X���ɰ�ɫ����������������������ǿ������������֪��ѡ��ˮ��Һ����Ҫ��
�ʴ�Ϊ��C��
��4��A-C��Ӧ����������������Һ�ķ�Ӧ����Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����H-I��Ӧ��������������������Ӧ�������������ķ�Ӧ����ѧ����ʽΪ��4Fe��0H��2+O2+2H2O�T4Fe��OH��3��
�ʴ�Ϊ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����4Fe��0H��2+O2+2H2O�T4Fe��OH��3��
��1�����������ж�FΪ����������ѧʽΪAl2O3��GΪ�Ȼ���������ѧʽFeCl2��
�ʴ�Ϊ��Al2O3��FeCl2��
��2��������������ķ������ô�������������
�ʴ�Ϊ���ô���������ĩ״���������������ڴ����ı��棻
��3��DΪAlCl3��EΪ��ɫ����˵������������������֤��XΪ����Һ��D��Eת���У�����������X���ɰ�ɫ����������������������ǿ������������֪��ѡ��ˮ��Һ����Ҫ��
�ʴ�Ϊ��C��
��4��A-C��Ӧ����������������Һ�ķ�Ӧ����Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����H-I��Ӧ��������������������Ӧ�������������ķ�Ӧ����ѧ����ʽΪ��4Fe��0H��2+O2+2H2O�T4Fe��OH��3��
�ʴ�Ϊ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����4Fe��0H��2+O2+2H2O�T4Fe��OH��3��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ���
���