��Ŀ����
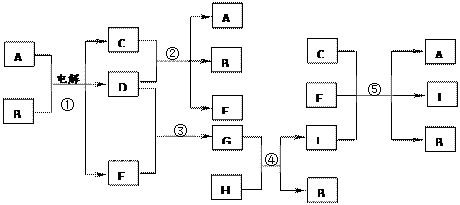
����ͼ��ʾ�����ʵ��ת���У���Ӧ���ڹ�ҵ�Ͽ���������������C����Ӧ���ڹ�ҵ�Ͽ�����������J��Na2FeO4������Ӧ�١��ڡ��ܺ͢ݾ�����ˮ��Һ�н��еķ�Ӧ�������£�D��E��G�������壬B����ɫҺ�壻F��ˮ��Һ����Ϊɱ����������H��һ������ʯ����Ҫ�ɷ֣���������Ԫ����ɣ���������Ԫ�ص���������Ϊ70%��
��ش��������⣺
��1��д��F�Ļ�ѧʽ�� ����2��д��G�ĵ���ʽ�� ��
��3����Ӧ�ٵĻ�ѧ����ʽΪ ��
���ǽ���Ӧ�����漰�Ļ�ѧ��ҵ��Ϊ ��
��4����Ӧ�ݵ����ӷ���ʽΪ ��
�������ƣ�Na2FeO4����������Ϊ��һ�֡���ɫ������Ч���ľ�ˮ������ԭ��Ϊ
��Na2FeO4����ǿ�����Կ�ɱ������
�� ��
 |
��ش��������⣺
��1��д��F�Ļ�ѧʽ�� ����2��д��G�ĵ���ʽ�� ��
��3����Ӧ�ٵĻ�ѧ����ʽΪ ��
���ǽ���Ӧ�����漰�Ļ�ѧ��ҵ��Ϊ ��
��4����Ӧ�ݵ����ӷ���ʽΪ ��
�������ƣ�Na2FeO4����������Ϊ��һ�֡���ɫ������Ч���ľ�ˮ������ԭ��Ϊ
��Na2FeO4����ǿ�����Կ�ɱ������
�� ��
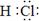 ��1��NaClO��2�֣���2�� ��2�֣�
��1��NaClO��2�֣���2�� ��2�֣���3��2NaCl + 2H2O �� H2�� + Cl2��+ 2NaOH ��2�֣� �ȼҵ��1�֣�
��4��2Fe3+ + 3ClO�D + 10OH�D �� 2FeO42�D + 3Cl�D + 5H2O��2�֣�
�ڱ�������ԭ��Fe3+������ˮ������Fe(OH)3��������ˮ�������Դﵽ����ˮ��Ŀ�ġ���1�֣�
A+B�������CDE�������ʣ�������NaCl��AgNO3��CuSO4����ˮ�ĵ�⣬D��E���������������ȷ���ǵ��NaCl��Һ����C��NaOH��F��NaClO��G��HCl ��H��һ������ʯ����Ҫ�ɷ֣���������Ԫ����ɣ���������Ԫ�ص���������Ϊ70%��H��Fe2O3,I��FeCl3����Ӧ�ݵ����ӷ���ʽΪ2Fe3+ + 3ClO�D + 10OH�D �� 2FeO42�D + 3Cl�D + 5H2O

��ϰ��ϵ�д�
�����Ŀ