��Ŀ����
��֪�����ڵ�����Ԫ��X��Y��Z��W��M��ԭ��������������X �dz������ʵ���ҪԪ�أ�Y ԭ�ӵ������ֻ��2�����ӣ�Z���ʿ��Ƴɰ뵼����ϣ�WԪ���γɵĵ���Ϊ��ɫ�Ĺ��壮��ش��������⣺
��1��Ԫ��W�����ڱ���λ��Ϊ ��
��2��X��Y�γɵĻ�����ĵ���ʽ ��
��3��Z�������ᄃ������Ϊ ��12gZ���������к��еĹ��ۼ���Ϊ ��
��4��X��W�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ���ĽṹʽΪ ��
��5��W��M���ǽϻ��õķǽ���Ԫ�أ���ʵ����ʵ����������Ԫ�صķǽ�����ǿ�� ���÷���ʽ��д������˵������
��1��Ԫ��W�����ڱ���λ��Ϊ
��2��X��Y�γɵĻ�����ĵ���ʽ
��3��Z�������ᄃ������Ϊ
��4��X��W�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ���ĽṹʽΪ
��5��W��M���ǽϻ��õķǽ���Ԫ�أ���ʵ����ʵ����������Ԫ�صķǽ�����ǿ��
�����������ڵ�����Ԫ��X��Y��Z��W��M��ԭ��������������X �dz������ʵ���ҪԪ�أ���X��NԪ�أ�Y ԭ�ӵ������ֻ��2��������ԭ����������X������Y��MgԪ�أ�Z���ʿ��Ƴɰ뵼�������Z��SiԪ�أ�WԪ���γɵĵ���Ϊ��ɫ�Ĺ��壬��W��SԪ�أ�M�Ƕ���������Ԫ�أ���ԭ����������W������M��ClԪ�أ����Ԫ�ء����ʵĽṹ�����ʷ������
����⣺�����ڵ�����Ԫ��X��Y��Z��W��M��ԭ��������������X �dz������ʵ���ҪԪ�أ���X��NԪ�أ�Y ԭ�ӵ������ֻ��2��������ԭ����������X������Y��MgԪ�أ�Z���ʿ��Ƴɰ뵼�������Z��SiԪ�أ�WԪ���γɵĵ���Ϊ��ɫ�Ĺ��壬��W��SԪ�أ�M�Ƕ���������Ԫ�أ���ԭ����������W������M��ClԪ�أ�
��1��W��SԪ�أ�SԪ�غ�����3�����Ӳ㣬�������6�����ӣ�����Ԫ�صĵ��Ӳ���������������ȣ�����������������������ȣ�����Sλ�ڵ������ڵ�VIA�壬
�ʴ�Ϊ���������ڵ�VIA�壻
��2��X��NԪ�أ�Y��MgԪ�أ������γɵĻ�������Mg3N2�������Ӻ�þ����֮��������Ӽ������������ʽΪ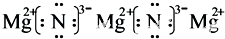 ��
��
�ʴ�Ϊ��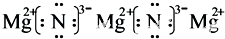 ��
��
��3��Z��SiԪ�أ��������辧�����乹������ԭ�ӣ���������Ϊ�ռ���״�ṹ��������ԭ�Ӿ��壬12g������������ʵ���=
=0.2mol��ÿ����ԭ�Ӻ���4��Si-O��������12gZ���������к��еĹ��ۼ���Ϊ0.8NA��
�ʴ�Ϊ��ԭ�Ӿ��壻0.8NA��
��4��N��Cl�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ������N2H4������HCl�����൱�ڰ��������е�һ����ԭ�ӱ�����ȡ�������ԼĽṹʽΪ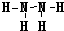 ��
��
�ʴ�Ϊ��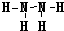 ��
��
��5��S��Cl���ǽϻ��õķǽ���Ԫ�أ�����������û������ʾ���֤����Ԫ�صķǽ����Դ�����Ԫ�أ���Ӧ����ʽΪ��Cl2+H2S=2HCl+S��
�ʴ�Ϊ��Cl2+H2S=2HCl+S��
��1��W��SԪ�أ�SԪ�غ�����3�����Ӳ㣬�������6�����ӣ�����Ԫ�صĵ��Ӳ���������������ȣ�����������������������ȣ�����Sλ�ڵ������ڵ�VIA�壬
�ʴ�Ϊ���������ڵ�VIA�壻
��2��X��NԪ�أ�Y��MgԪ�أ������γɵĻ�������Mg3N2�������Ӻ�þ����֮��������Ӽ������������ʽΪ
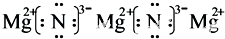 ��
���ʴ�Ϊ��
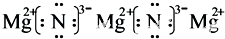 ��
����3��Z��SiԪ�أ��������辧�����乹������ԭ�ӣ���������Ϊ�ռ���״�ṹ��������ԭ�Ӿ��壬12g������������ʵ���=
| 12g |
| 60g/mol |
�ʴ�Ϊ��ԭ�Ӿ��壻0.8NA��
��4��N��Cl�γɵ��⻯��ֱ�Ϊ���ң��Ҽס��������ĵ�������ȣ������N2H4������HCl�����൱�ڰ��������е�һ����ԭ�ӱ�����ȡ�������ԼĽṹʽΪ
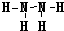 ��
���ʴ�Ϊ��
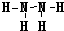 ��
����5��S��Cl���ǽϻ��õķǽ���Ԫ�أ�����������û������ʾ���֤����Ԫ�صķǽ����Դ�����Ԫ�أ���Ӧ����ʽΪ��Cl2+H2S=2HCl+S��
�ʴ�Ϊ��Cl2+H2S=2HCl+S��
���������⿼����Ԫ��λ�ýṹ���ʵĹ�ϵ����ȷ�ƶ�Ԫ���ǽⱾ��ؼ����ѵ��ǵ���þ����ʽ����д���������辧����Si-O���ļ��㣬����֪ʶǨ�Ƶķ�����д�µĽṹʽ���Ѷ��еȣ�

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
�����Ŀ

 HSO3-+OH-
HSO3-+OH-
