��Ŀ����
��16�֣���1��0.02mol/L��CH3COOH��Һ��0.02mol/L CH3COONa��Һ�������ϣ���֪�û����Һ�У�c(H+)>c(OH-)���á�>��<��=��������գ�
����Һ��c(Na+)_____c(CH3COO-) �� c(CH3COO-)_______ c(CH3COOH)
�� c(CH3COOH)+c(CH3COO-) 0.04 mol/L
��2����HnA���B(OH)m��ȫ��Ӧ��������.
����HnAΪHCl���Ҹ�����Һ��pH��7�������ӷ���ʽ˵��ԭ��
������0.4mol��L��1��NaOH��Һ��0.2mol��L��1��HnA��Һ�������Ϻ�pH=10��
��HnAΪ ������ţ�.
a.һԪǿ�� b. һԪ���� c. ��Ԫǿ�� d. ��Ԫ����
��3��ijѧУ������ȤС��Ӻ�ˮɹ�κ����±(��Ҫ��Na+��Mg2+��Cl����Br����)��ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺
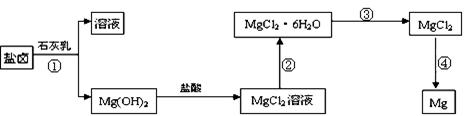
��.�ӹ��̢ٵõ���Mg(OH)2�����л���������Ca(OH)2 ����ȥ����Ca(OH)2�ķ������Ƚ��������뵽ʢ�� ��Һ���ձ��У���ֽ�����ˡ�ϴ�ӿɵô�����Mg(OH)2��
��.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
��.д�����̢��з�����Ӧ�Ļ�ѧ����ʽ
����Һ��c(Na+)_____c(CH3COO-) �� c(CH3COO-)_______ c(CH3COOH)
�� c(CH3COOH)+c(CH3COO-) 0.04 mol/L
��2����HnA���B(OH)m��ȫ��Ӧ��������.
����HnAΪHCl���Ҹ�����Һ��pH��7�������ӷ���ʽ˵��ԭ��
������0.4mol��L��1��NaOH��Һ��0.2mol��L��1��HnA��Һ�������Ϻ�pH=10��
��HnAΪ ������ţ�.
a.һԪǿ�� b. һԪ���� c. ��Ԫǿ�� d. ��Ԫ����
��3��ijѧУ������ȤС��Ӻ�ˮɹ�κ����±(��Ҫ��Na+��Mg2+��Cl����Br����)��ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺

��.�ӹ��̢ٵõ���Mg(OH)2�����л���������Ca(OH)2 ����ȥ����Ca(OH)2�ķ������Ƚ��������뵽ʢ�� ��Һ���ձ��У���ֽ�����ˡ�ϴ�ӿɵô�����Mg(OH)2��
��.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
��.д�����̢��з�����Ӧ�Ļ�ѧ����ʽ
��16�֣�(1)�� < ��2�֣� �� > ��2�֣� �� < ��2�֣�
��2���� Bm+��mH2O B(OH)m��mH�� ��2�֣� �� d ��2�֣�
B(OH)m��mH�� ��2�֣� �� d ��2�֣�
(3) i. MgCl2(���Ȼ�þ) ��2�֣�
ii. HCl����MgCl2��ˮ�⣬����ˮ��������2�֣�
iii.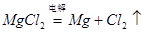 ��2�֣�
��2�֣�
��2���� Bm+��mH2O
 B(OH)m��mH�� ��2�֣� �� d ��2�֣�
B(OH)m��mH�� ��2�֣� �� d ��2�֣�(3) i. MgCl2(���Ȼ�þ) ��2�֣�
ii. HCl����MgCl2��ˮ�⣬����ˮ��������2�֣�
iii.
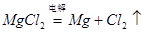 ��2�֣�
��2�֣���

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
 Fe3+��NO3-��SO42-
Fe3+��NO3-��SO42-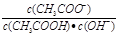 ����
����